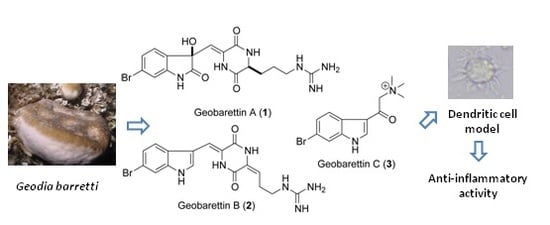
6-Bromoindole Derivatives from the Icelandic Marine Sponge Geodia barretti: Isolation and Anti-Inflammatory Activity
Abstract
:1. Introduction
2. Results
2.1. Structural Elucidation
2.2. Anti-Inflammatory Activity
3. Discussion
4. Materials and Methods
4.1. General Produres
4.2. Animal Materials
4.3. Extraction and Isolation
4.4. Hydrogenolysis of R-Dioxindole (8a) to (R)-3-Propyldioxindole (8)
4.5. Hydrogenolysis of Geobarrettin A (1) to Debromodihydrogeobarrettin A (1a)
4.6. Acid Hydrolysis of Debromodihydrogeobarrettin B (1a)
4.7. Absolute Configuration of the Amino Acid of Geobarrettin A (1)
4.8. Absolute Configuration of the Amino Acid of Barettin (4)
4.9. Maturation and Activation of DCs
4.10. Co-Culture of DCs and Allogeneic CD4+ T Cells
4.11. Determination of Cytokine Concentration by ELISA
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dubois, R.N. The Jeremiah Metzger Lecture: Inflammation, immune modulators, and chronic disease. Trans. Am. Clin. Climatol. Assoc. 2015, 126, 230–236. [Google Scholar] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.S.; Rodriguez, A.D.; Taglialatela-Scafati, O.; Fusetani, N. Marine pharmacology in 2012–2013: Marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis, and antiviral activities; Affecting the immune and nervous systems, and other miscellaneous mechanisms of action. Mar. Drugs 2017, 15, 273. [Google Scholar] [CrossRef]
- Malve, H. Exploring the ocean for new drug developments: Marine pharmacology. J. Pharm. Bioallied. Sci. 2016, 8, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, K.; Kim, S.K. Marine invertebrate natural products for anti-inflammatory and chronic diseases. Evid. Based Complement. Alternat. Med. 2013, 2013, 572859. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Wahlqvist, M.L.; He, G.; Yang, M.; Li, D. Natural products and anti-inflammatory activity. Asia Pac. J. Clin. Nutr. 2006, 15, 143–152. [Google Scholar] [PubMed]
- Gonzalez, Y.; Torres-Mendoza, D.; Jones, G.E.; Fernandez, P.L. Marine diterpenoids as potential anti-inflammatory agents. Mediat. Inflamm. 2015, 2015, 263543. [Google Scholar] [CrossRef] [PubMed]
- Keyzers, R.A.; Davies-Coleman, M.T. Anti-inflammatory metabolites from marine sponges. Chem. Soc. Rev. 2005, 34, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Lidgren, G.; Bohlin, L. Studies of Swedish marine organisms. 7. A novel biologically-active indole alkaloid from the sponge Geodia baretti. Tetrahedron Lett. 1986, 27, 3283–3284. [Google Scholar] [CrossRef]
- Solter, S.; Dieckmann, R.; Blumenberg, M.; Francke, W. Barettin, revisited? Tetrahedron Lett. 2002, 43, 3385–3386. [Google Scholar] [CrossRef]
- Sjogren, M.; Goransson, U.; Johnson, A.L.; Dahlstrom, M.; Andersson, R.; Bergman, J.; Jonsson, P.R.; Bohlin, L. Antifouling activity of brominated cyclopeptides from the marine sponge Geodia barretti. J. Nat. Prod. 2004, 67, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Hedner, E.; Sjogren, M.; Hodzic, S.; Andersson, R.; Goransson, U.; Jonsson, P.R.; Bohlin, L. Antifouling activity of a dibrominated cyclopeptide from the marine sponge Geodia barretti. J. Nat. Prod. 2008, 71, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Olsen, E.K.; Hansen, E.; Moodie, W.; Isaksson, J.; Sepcic, K.; Cergolj, M.; Svenson, J.; Andersen, J.H. Marine AChE inhibitors isolated from Geodia barretti: Natural compounds and their synthetic analogs. Org. Biomol. Chem. 2016, 14, 1629–1640. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Tanaka, N.; Kubota, T.; Ishiyama, H.; Shibazaki, A.; Gonoi, T.; Fromont, J.; Kobayashi, J. Heteroaromatic alkaloids, nakijinamines, from a sponge Suberites sp. Tetrahedron 2012, 68, 8545–8550. [Google Scholar] [CrossRef]
- Lidgren, G.; Bohlin, L.; Christophersen, C. Studies of Swedish marine organisms .10. Biologically-active compounds from the marine sponge Geodia barretti. J. Nat. Prod. 1988, 51, 1277–1280. [Google Scholar] [CrossRef]
- Carstens, B.B.; Rosengren, K.J.; Gunasekera, S.; Schempp, S.; Bohlin, L.; Dahlstrom, M.; Clark, R.J.; Goransson, U. Goransson, U. Isolation, characterization, and synthesis of the barrettides: Disulfide-containing peptides from the marine sponge Geodia barretti. J. Nat. Prod. 2015, 78, 1886–1893. [Google Scholar] [CrossRef] [PubMed]
- Hougaard, L.; Christophersen, C.; Nielsen, P.H.; Klitgaard, A.; Tendal, O. The chemical-composition of species of Geodia, Isops and Stryphnus (Choristida, Demospongia, Porifera)–a comparative-study with some taxonomical implications. Biochem. Syst. Ecol. 1991, 19, 223–235. [Google Scholar] [CrossRef]
- Johnson, A.L.; Bergman, J.; Sjogren, M.; Bohlin, L. Synthesis of barettin. Tetrahedron 2004, 60, 961–965. [Google Scholar] [CrossRef]
- Borthwick, A.D. 2,5-Diketopiperazines: Synthesis, reactions, medicinal chemistry, and bioactive natural products. Chem. Rev. 2012, 112, 3641–3716. [Google Scholar] [CrossRef] [PubMed]
- Lind, K.F.; Hansen, E.; Osterud, B.; Eilertsen, K.E.; Bayer, A.; Engqvist, M.; Leszczak, K.; Jorgensen, T.O.; Andersen, J.H. Antioxidant and anti-inflammatory activities of barettin. Mar. Drugs 2013, 11, 2655–2666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedner, E.; Sjogren, M.; Frandberg, P.A.; Johansson, T.; Goransson, U.; Dahlstrom, M.; Jonsson, P.; Nyberg, F.; Bohlin, L. Brominated cyclodipeptides from the marine sponge Geodia barretti as selective 5-HT ligands. J. Nat. Prod. 2006, 69, 1421–1424. [Google Scholar] [CrossRef] [PubMed]
- Pooley, J.L.; Heath, W.R.; Shortman, K. Cutting edge: Intravenous soluble antigen is presented to CD4 T cells by CD8- dendritic cells, but cross-presented to CD8 T cells by CD8+ dendritic cells. J. Immunol. 2001, 166, 5327–5330. [Google Scholar] [CrossRef] [PubMed]
- Manetti, R.; Gerosa, F.; Giudizi, M.G.; Biagiotti, R.; Parronchi, P.; Piccinni, M.P.; Sampognaro, S.; Maggi, E.; Romagnani, S.; Trinchieri, G.; et al. Interleukin 12 induces stable priming for interferon gamma (IFN-gamma) production during differentiation of human T helper (Th) cells and transient IFN-gamma production in established Th2 cell clones. J. Exp. Med. 1994, 179, 1273–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyer, S.S.; Cheng, G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, N.; Ueno, H. Regulation of human helper T cell subset differentiation by cytokines. Curr. Opin. Immunol. 2015, 34, 130–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Workman, C.J.; Szymczak-Workman, A.L.; Collison, L.W.; Pillai, M.R.; Vignali, D.A. The development and function of regulatory T cells. Cell Mol. Life Sci. 2009, 66, 2603–2622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kupchan, S.M.; Tsou, G.; Sigel, C.W. Datiscacin, a novel cytotoxic cucurbitacin 20-acetate from Datisca glomerata. J. Org. Chem. 1973, 38, 1420–1421. [Google Scholar] [CrossRef] [PubMed]
- Vanwagenen, B.C.; Larsen, R.; Cardellina, J.H.; Randazzo, D.; Lidert, Z.C.; Swithenbank, C. Ulosantoin, a potent insecticide from the sponge Ulosa ruetzleri. J. Org. Chem. 1993, 58, 335–337. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, F.J.; Wang, Y.M.; Li, H.J. Dereplication-guided isolation of novel hepatoprotective triterpenoid saponins from Celosiae semen by high-performance liquid chromatography coupled with electrospray ionization tandem quadrupole-time-of-flight mass spectrometry. J. Pharm. Biomed. Anal. 2017, 132, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.G.; Li, A.; Yan, B.C.; Niu, S.B.; Tang, J.W.; Li, X.N.; Du, X.; Challis, G.L.; Che, Y.; Sun, H.D.; et al. LC-MS-guided isolation of penicilfuranone A: A new antifibrotic furancarboxylic acid from the plant endophytic fungus Penicillium sp. sh18. J. Nat. Prod. 2016, 79, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Di, Y.T.; Wang, Y.H.; Zhang, Z.; Mu, S.Z.; Fang, X.; Zhang, Y.; Tan, C.J.; Zhang, Q.; Yan, X.H.; et al. Gelegamines A-E: Five new oxindole alkaloids from Gelsemium elegans. Tetrahedron 2009, 65, 4551–4556. [Google Scholar] [CrossRef]
- Kamano, Y.; Zhang, H.P.; Ichihara, Y.; Kizu, H.; Komiyama, K.; Pettit, G.R. Convolutamydine A, a novel bioactive hydroxyoxindole alkaloid from marine bryozoan Amathia Convoluta. Tetrahedron Lett. 1995, 36, 2783–2784. [Google Scholar] [CrossRef]
- Wu, H.; Xue, F.; Xiao, X.; Qin, Y. Total synthesis of (+)-perophoramidine and determination of the absolute configuration. J. Am. Chem. Soc. 2010, 132, 14052–14054. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Saravanan, S.; Gupta, N.; Abdi, S.H.R.; Khan, N.U.; Kureshy, R.I.; Bajaj, H.C. Phosphotungstic acid as an efficient catalyst for allylation of isatins and N-tert-butyloxycarbonylamido sulfones under solvent-free conditions. Asian J. Org. Chem. 2014, 3, 1173–1181. [Google Scholar] [CrossRef]
- Wei, X.; Henriksen, N.M.; Skalicky, J.J.; Harper, M.K.; Cheatham, T.E., 3rd; Ireland, C.M.; Van Wagoner, R.M. Araiosamines A-D: Tris-bromoindole cyclic guanidine alkaloids from the marine sponge Clathria (Thalysias) araiosa. J. Org. Chem. 2011, 76, 5515–5523. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.Q.; Sattler, I.; Thiericke, R.; Grabley, S.; Feng, X.Z. Maremycins C and D, new diketopiperazines, and maremycins E and F, novel polycyclic spiro-indole metabolites isolated from Streptomyces sp. Eur. J. Org. Chem. 2001, 261–267. [Google Scholar] [CrossRef]
- Hanhan, N.V.; Sahin, A.H.; Chang, T.W.; Fettinger, J.C.; Franz, A.K. Catalytic asymmetric synthesis of substituted 3-hydroxy-2-oxindoles. Angew. Chem. Int. Ed. Engl. 2010, 49, 744–747. [Google Scholar] [CrossRef] [PubMed]
- Salib, M.N.; Molinski, T.F. Six trikentrin-like cyclopentanoindoles from trikentrion flabelliforme. Absolute structural assignment by NMR and ECD. J. Org. Chem. 2018, 83, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Sonderegger, O.J.; Burgi, T.; Limbach, L.K.; Baiker, A. Enantio selective reduction of isatin derivatives over cinchonidine modified Pt/alumina. J. Mol. Catal. A Chem. 2004, 217, 93–101. [Google Scholar] [CrossRef]
- Phyo, Y.Z.; Ribeiro, J.; Fernandes, C.; Kijjoa, A.; Pinto, M.M.M. Marine natural peptides: Determination of absolute configuration using liquid chromatography methods and evaluation of bioactivities. Molecules 2018, 23, 306. [Google Scholar] [CrossRef] [PubMed]
- Salib, M.N.; Molinski, T.F. Cyclic hexapeptide dimers, antatollamides A and B, from the ascidian Didemnum molle. A tryptophan-derived auxiliary for l- and d-amino acid assignments. J. Org. Chem. 2017, 82, 10181–10187. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, K.; Benner, R. Hydrolysis-induced racemization of amino acids. Limnol. Oceanogr.-Meth. 2005, 3, 318–325. [Google Scholar] [CrossRef] [Green Version]
- Albinsson, B.; Norden, B. Excited-state properties of the indole chromophore: Electronic-transition moment directions from linear dichroism measurements: Effect of methyl and methoxy substituents. J. Phys. Chem. 1992, 96, 6204–6212. [Google Scholar] [CrossRef]
- Shin, C.G.; Hayakawa, M.; Mikami, K.; Yoshimura, J. Syntheses and configurational assignments of albonoursin and its three geometric isomers. Tetrahedron Lett. 1977, 18, 863–866. [Google Scholar] [CrossRef]
- Shin, C.; Hayakawa, M.; Suzuki, T.; Ohtsuka, A.; Yoshimura, J. α,β-Unsaturated carboxylic-acid derivatives. 13. Synthesis andconfiguration of alkyl 2-acylamino-2-alkenoates and their cyclized 2,5-piperazinedione derivatives. Bull. Chem. Soc. Jpn. 1978, 51, 550–554. [Google Scholar] [CrossRef]
- Kasheverov, I.E.; Shelukhina, I.V.; Kudryavtsev, D.S.; Makarieva, T.N.; Spirova, E.N.; Guzii, A.G.; Stonik, V.A.; Tsetlin, V.I. 6-Bromohypaphorine from marine nudibranch mollusk Hermissenda crassicornis is an agonist of human α7 nicotinic acetylcholine receptor. Mar. Drugs 2015, 13, 1255–1266. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Nishi, J.; Ishibashi, M.; Kobayashi, J. Two new tryptophan-derived alkaloids from the Okinawan marine sponge Aplysina sp. J. Nat. Prod. 1994, 57, 1008–1011. [Google Scholar] [CrossRef] [PubMed]
- Corthay, A. How do regulatory T cells work? Scand. J. Immunol. 2009, 70, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, S. Th1 and Th2 in human diseases. Clin. Immunol. Immunopathol. 1996, 80, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Gee, K.; Guzzo, C.; Che Mat, N.F.; Ma, W.; Kumar, A. The IL-12 family of cytokines in infection, inflammation and autoimmune disorders. Inflamm. Allergy Drug Targets 2009, 8, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Pence, H.E.; Williams, A. ChemSpider: An online chemical information resource. J. Chem. Educ. 2010, 87, 1123–1124. [Google Scholar] [CrossRef]
- Freysdottir, J.; Sigurpalsson, M.B.; Omarsdottir, S.; Olafsdottir, E.S.; Vikingsson, A.; Hardardottir, I. Ethanol extract from birch bark (Betula pubescens) suppresses human dendritic cell mediated Th1 responses and directs it towards a Th17 regulatory response in vitro. Immunol. Lett. 2011, 136, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Di, X.; Oskarsson, J.T.; Omarsdottir, S.; Freysdottir, J.; Hardardottir, I. Lipophilic fractions from the marine sponge Halichondria sitiens decrease secretion of pro-inflammatory cytokines by dendritic cells and decrease their ability to induce a Th1 type response by allogeneic CD4+ T cells. Pharm. Biol. 2017, 55, 2116–2122. [Google Scholar] [CrossRef] [PubMed]
- Iso, H.; Cui, R.; Date, C.; Kikuchi, S.; Tamakoshi, A.; Group, J.S. C-reactive protein levels and risk of mortality from cardiovascular disease in Japanese: The JACC Study. Atherosclerosis 2009, 207, 291–297. [Google Scholar] [CrossRef] [PubMed]







| No. | 1 | 2 | 3 | ||||
|---|---|---|---|---|---|---|---|
| δH, Mult. (J in Hz) a | δH, Mult. (J in Hz) b,c | δC, Type a | δH, Mult. (J in Hz) a | δC, Type a | δH, Mult. (J in Hz) a | δC, Type a | |
| 1 | 10.76, br s | ||||||
| 2 | 179.3, C | 7.83, s | 127.6, CH | 8.27, s | 135.9, CH | ||
| 3 | 78.3, C | 110.0, C | 116.1, C | ||||
| 3a | 132.4, C | 127.5, C | 125.7, C | ||||
| 4 | 7.25, br s | 7.26, d (7.9) | 126.9, CH | 7.61, d (8.5) | 120.9, CH | 8.16, d (8.5) | 124.1, CH |
| 5 | 7.25, br s | 7.23, dd (7.9, 1.5) | 127.1, CH | 7.27, dd (8.5, 2.0) | 124.7, CH | 7.39, dd (8.5, 2.0) | 127.1, CH |
| 6 | 124.7, C | 117.2, C | 118.3, C | ||||
| 7 | 7.11, br s | 7.03, d (1.5) | 115.0, CH | 7.62, d (2.0) | 115.7, CH | 7.68, d (2.0) | 116.3, CH |
| 7a | 143.9, C | 138.5, C | 139.2, C | ||||
| 8 | 5.61, s | 5.37, s | 112.8, CH | 7.22, s | 111.0, CH | 186.0, C | |
| 9 | 132.2, C | 123.5, C | 4.92, s | 67.8, CH2 | |||
| 10 | 9.88, s | ||||||
| 11 | 167.2, C | 159.3, C | 3.42, s | 54.9, CH3 | |||
| 12 | 4.28, t (6.0) | 4.19, td (5.4, 1.9) | 56.0, CH | 130.5, C | 3.42, s | 54.9, CH3 | |
| 13 | 8.58, d (1.9) | 3.42, s | 54.9, CH3 | ||||
| 14 | 160.5, C | 160.3, C | |||||
| 15 | 1.89, m; 2.01, m | 1.76, m | 32.6, CH2 | 5.98, t (7.8) | 114.9, CH | ||
| 16 | 1.67, m; 1.74, m | 1.49, m; 1.55, m | 24.8, CH2 | 2.57, q (7.8) | 26.2, CH2 | ||
| 17 | 3.24, t (6.0) | 3.12, m | 42.0, CH2 | 3.37, t (7.2) | 41.4, CH2 | ||
| 18 | 7.49, br t (5.5) | ||||||
| 19 | 158.7, C | 158.8, C | |||||
| 20,21 | 6.60–7.65, br | ||||||
| 3-OH | 7.28, br s | ||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di, X.; Rouger, C.; Hardardottir, I.; Freysdottir, J.; Molinski, T.F.; Tasdemir, D.; Omarsdottir, S. 6-Bromoindole Derivatives from the Icelandic Marine Sponge Geodia barretti: Isolation and Anti-Inflammatory Activity. Mar. Drugs 2018, 16, 437. https://doi.org/10.3390/md16110437
Di X, Rouger C, Hardardottir I, Freysdottir J, Molinski TF, Tasdemir D, Omarsdottir S. 6-Bromoindole Derivatives from the Icelandic Marine Sponge Geodia barretti: Isolation and Anti-Inflammatory Activity. Marine Drugs. 2018; 16(11):437. https://doi.org/10.3390/md16110437
Chicago/Turabian StyleDi, Xiaxia, Caroline Rouger, Ingibjorg Hardardottir, Jona Freysdottir, Tadeusz F. Molinski, Deniz Tasdemir, and Sesselja Omarsdottir. 2018. "6-Bromoindole Derivatives from the Icelandic Marine Sponge Geodia barretti: Isolation and Anti-Inflammatory Activity" Marine Drugs 16, no. 11: 437. https://doi.org/10.3390/md16110437