Abstract
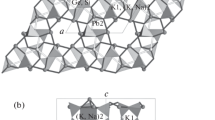
The crystal structure of Pb6O[(Si6Al2)O20)] is investigated using X-ray diffraction. The compound has tetragonal symmetry, space group I4/mmm, a = 11.7162(10) Å, c = 8.0435(12) Å, and V = 1104.13(2) Å3. The structure is refined to R 1 = 0.036 for 562 unique reflections with [F 0] ≥ 4σF. The structure contains two symmetrically independent positions of the Pb2+ cations coordinated by five O atoms (Pb2+-O2− = 2.34–2.68 Å). The TO4 tetrahedra (T = Si, Al) form tubular [(Si6Al2)O20] chains extended along the c axis. The O4 oxygen atom is not bonded to the Si and Al atoms and is octahedrally coordinated by six Pb atoms with the formation of an oxo-centered OPb6 octahedron. The assumption is made that, in some of lead silicate and aluminosilicate glasses, a number of oxygen atoms are located outside the tetrahedral structure and represent segregation centers of the Pb2+ cations due to the formation of oxo-centered complexes.
Similar content being viewed by others
References
Pronkin, A.A., Kogan, V.E., Sokolov, I.A., and Tarlakov, Yu.P., Electrical Properties and Structure of Fluorine-Containing Lead Silicate Glasses, Fiz. Khim. Stekla, 1995, vol. 21, no. 5, pp. 496–506 [Glass Phys. Chem. (Engl. transl.), 1995, vol. 21, no, 21, no. 5, pp. 357–363].
Kabanov, V.O., Podol’skaya, T.M., and Yanush, O.V., Raman Spectra and Structure of the PbO-SiO2 Glasses, Fiz. Khim. Stekla, 1996, vol. 22, no. 1, pp. 25–33 [Glass Phys. Chem. (Engl. transl.), 1996, vol. 22, no. 1, pp. 19–26].
Sokolov, I.A., Murin, I.V., Wiemhöfer, H.-D., and Pronkin, A.A., The Nature of Electric Conductivity in the PbO-SiO2 Glasses, Fiz. Khim. Stekla, 1998, vol. 24, no. 2, pp. 158–167 [Glass Phys. Chem. (Engl. transl.), 1998, vol. 24, no. 2, pp. 112–118].
Mylyanych, A.O., Sheredko, M.A., and Melnyk, S.K., Study of Glass Structures and Crystalline Phases in the PbO-Al2O3-SiO2 System, J. Anal. At. Spectrom., 1999, vol. 14, pp. 513–521.
Jeon, Y.-W., Kim, M.-J., Kwon, O.-S., Lee, J.-S., and Ryu, B.-K., Fabrication of Glass Passivation Films for Power Devices by Screen Printing and Their Characterization, J. Ceram. Soc. Jpn., 2004, vol. 112, no. 1305, p. 1245–1249.
Prabakar, S. and Rao, K.J., MAS NMR Studies of Ternary Silicate Glasses, Philos. Mag. B, 1991, vol. 64, no. 4, pp. 401–411.
Downs, R.T., Hazen, R.M., Finger, L.W., and Gasparik, T., Crystal Chemistry of Lead Aluminosilicate Hollandite: A New High-Pressure Synthetic Phase with Octahedral Si, Am. Mineral., 1995, vol. 80, pp. 937–940.
Benna, P., Tribaudino, M., and Bruno, E., The Structure of Ordered and Disordered Lead Feldspar (PbAl2Si2O8), Am. Mineral., 1996, vol. 81, pp. 1337–1343.
Pentinghaus, H., Crystal Chemistry of Hollandites A x M 8(O,OH)16(x≤2), Phys. Chem. Miner., 1978, vol. 3, pp. 85–86.
Chen, S., Zhao, B., Hayes, P.C., and Jak, E., Experimental Study of Phase Equilibria in the PbO-Al2O3-SiO2 System, Metall. Mater. Trans. B, 2001, vol. 32, pp. 997–1005.
Krivovichev, S.V. and Brown, I.D., Are the Compressive Effects of Encapsulation an Artifact of the Bond Valence Parameters? Z. Kristallogr., 2001, vol. 216, pp. 245–247.
Brese, N.E. and O’Keeffe, M., Bond-Valence Parameters for Solids, Acta Crystallogr., Sect. B: Struct. Sci., 1991, vol. 47, pp. 192–197.
Krivovichev, S.V., Minerals with Antiperovskite Structure: A Review, Z. Kristallogr., 2008, vol. 223, pp. 109–113.
Moore, P.B., Sen Gupta, P.K., and Schlemper, E.O., Solid Solution in Plumbous Potassium Oxysilicate Affected by Interaction of a Lone Pair with Bond Pairs, Nature (London), 1985, vol. 318, pp. 548–550.
Peacor, D.R. and Buerger, M.J., The Determination and Refinement of the Structure of Narsarsukite, Na2TiO[Si4O10], Am. Mineral., 1962, vol. 47, pp. 539–556.
Zhang, D.-S., Ma, B.-Q., Jin, T.-Z., Gao, S., Yan, C.-H., and Mak, T.C.W., Oxo-Centered Regular Octahedral Lanthanide Clusters, New J. Chem., 2000, vol. 24, pp. 61–62.
Schleid, T. and Wontcheu, J., Lanthanoid(III) Oxide Chloride Oxoselenates(IV): A Fascinating Class of Multinary Compounds, J. Alloys Compd., 2006, vol. 418, pp. 45–52.
Lissner, F. and Schleid, T., Cs2Gd6N2Te7: The First Quaternary Nitride Telluride of the Lanthanides, J. Alloys Compd., 2006, vol. 418, pp. 68–72.
Krivovichev, S.V. and Filatov, S.K., Kristallokhimiya mineralov i neorganicheskikh soedinenii s kompleksami aniontsentrirovannykh tetraedrov (Crystal Chemistry of Minerals and Inorganic Compounds with Complexes of Anion-Centered Tetrahedra), St. Petersburg: St. Petersburg State University, 2001 [in Russian].
Rao, D.G. and Rao, K.J., X-Ray Diffraction Study of Lead Oxide-Lead Chloride Glasses, Phys. Chem. Glasses, 1984, vol. 25, pp. 11–15.
Rao, B.G., Rao, K.J., and Wong, J., L-Edge EXAFS Studies of the Coordination of Lead in PbO-PbF2 Glasses, J. Chem. Soc., Faraday Trans. 1, 1988, vol. 84, pp. 1773–1778.
Siidra, O.I., Krivovichev, S.V., and Depmaier, V., Crystal Chemistry of Natural and Synthetic Lead Oxohalides: I. Crystal Structure of a New Oxochloride, Pb13O10Cl6, Zap. Ross. Mineral. O-va, 2007, vol. 136, no. 1, pp. 79–89.
Siidra, O.I., Krivovichev, S.V., and Depmeier, W., Structure and Mechanism of the Ionic Conductivity of the Nonstoichiometric Compound Pb2 + x OCl2 + 2x , Dokl. Akad. Nauk, 2007, vol. 414, no. 4 pp. 501–504 [Dokl. Phys. Chem. (Engl. transl.), 2007, vol. 414, part 2, pp. 128–131].
Siidra, O.I., Krivovichev, S.V., Armbruster, T., and Depmeier, W., Crystal Chemistry of the Mendipite-Type System Pb3O2Cl2-Pb3O2Br2, Z. Kristallogr., 2008, vol. 223, pp. 204–211.
Krivovicehv, S.V., Siidra, O.I., Nazarchuk, E.V., Burns, P.C., and Depmeier, W., Particular Topological Complexity of Lead Oxide Blocks in Pb31O22 X 18 (X = Br, Cl), Inorg. Chem., 2006, vol. 45, pp. 3846–3848.
Lusvardi, G., Malavasi, G., Cortada, M., Menabue, L., Menziani, M.C., Pedone, A., and Segre, U., Elucidation of the Structural Role of Fluorine in Potentially Bioactive Glasses by Experimental and Computational Investigation, J. Phys. Chem. B, 2008, vol. 112, pp. 12730–12739.
Kato, K., Die Kristallstruktur des Bleisilicats Pb11Si3O17, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1982, vol. 38, pp. 57–62.
Dent Glasser, L.S., Howie, R.A., and Smart, R.M., The Structure of Lead ‘Orthosilicate,’ 2PbO · SiO2, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1981, vol. 37, pp. 303–306.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © O.I. Siidra, S.V. Krivovichev, W. Depmeier, 2009, published in Fizika i Khimiya Stekla.
Rights and permissions
About this article
Cite this article
Siidra, O.I., Krivovichev, S.V. & Depmeier, W. Crystal structure of Pb6O[(Si6Al2)O20]. Glass Phys Chem 35, 406–410 (2009). https://doi.org/10.1134/S1087659609040099
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1087659609040099