Abstract
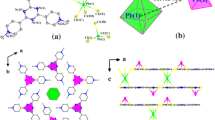
Crystals of lead oxobromide Pb7O4(OH)4Br2 have been synthesized by hydrothermal method. The structure of the new compound has been studied with X-ray single-crystal diffraction analysis. The compound is monoclinic, space group C1121; unit-cell dimensions are a = 5.852(4), b = 13.452(7), c = 19.673(9) Å, γ = 90.04°, V = 1548.7(15) Å3. The structure has been solved by direct methods and refined to R 1 = 0.1138 for 1847 observed Pb7O4(OH)4Br2 unique reflections. The structure contains seven symmetrically independent bivalent Pb atoms. The coordination polyhedrons of Pb are strongly distorted due to stereochemical activity of unshared electron pair 6s 2. Oxygen atoms are tetrahedrally coordinated by four Pb2+ cations with the formation of oxocentered tetrahedrons OPb4. The compound is based on [O2Pb3]2+ double chains formed by OPb4 tetrahedrons. (OH)Pb2 dimers combine the [O2Pb3]2+ chains into 3D framework. Channels in the framework are parallel to [100] and are occupied by Br anions.
Similar content being viewed by others
References
N. E. Brese and M. Keeffe, “Bond-Valence Parameters for Solids,” Acta Crystallogr. B47, 192–197 (1991).
R. Edwards, R. D. Gillard, P. A. Williams, and A. M. Pollard, “Studies of Secondary Mineral Formation in the PbO-H2O-HCl System,” Mineral. Mag. 57, 53–65 (1992).
J. J. Finney, E. J. Graeber, and A. Rosenzweig, “The Structure of Chloroxiphite, Pb3CuO2(OH)2Cl2,” Mineral. Mag. 41, 457–361 (1997).
M. Kiyama, K. Murakami, T. Takada, et al., “Formation and Solubility of Basic Lead Chlorides at Different PH Values” (Chem. Lett., 1976), pp. 23–28
V. Kramer and E. Post, “Preparation and Structural Characterization of the Lead Oxide Iodide Pb3O2I2,” Mater. Res. Bull. 20, 407–412 (1985).
S. V. Krivovichev and I. D. Brown, “Are the Compressive Effects of Encapsulation an Artifact of the Bond Valence Parameters?,” Zschr. Kristallogr. 216, 245–247 (2001).
S. V. Krovovichev and P. C. Burns, “Crystal Chemistry of Lead Oxide Chlorides. I. Crystal Structures of Synthetic Mendipite, Pb3O2Cl2, and Synthetic Damaraite, Pb3O2(OH)Cl,” Eur. J. Mineral. 13, 801–809 (2001a).
S. V. Krivovichev and P. C. Burns, “Crystal Structure of Pb3O2(OH)Br, a Br-Analogue of Damaraite,” Solid State Sci. 3, 455–459 (2001b).
S. V. Krivovichev and P. C. Burns, “Crystal Chemistry of Lead Oxide Chlorides. I. Crystal Structure of Pb2O2(OH)4Cl2,” Eur. J. Mineral. 14, 135–139 (2002).
S. V. Krivovichev and S. K. Filatov, Crystal Chemistry of Minerals and Inorganic Compounds with Complexes of Anion-Centered Tetrahedrons (St.-Petersburg State Univ., St. Petersburg, 2001) [in Russian].
S. V. Krivovichev, E. Yu. Avdontseva, and P. C. Burns, “Synthesis and Crystal Structure of Pb3O2(SeO3),” Zschr. Anorg. Allg. Chemie 630, 558–562 (2004).
L. Noren, E. S. Q. Tan, R. L. Withers, et al., “Neutron, X-ray, and Electron Diffraction Study of Structures of Pb3O2X2 (X = Cl, Br),” Mater. Res. Bull. 37, 1431–1442 (2002).
O. I. Siidra, S. V. Krivovichev, T. Armbruster, and W. Depmeier, “Lead-Earth Oxyhalides: Syntheses and Characterization of Pb6LaO7X (X = Cl, Br),” Inorg. Chem. 46, 1523–1525 (2007).
O. I. Siidra, S. V. Krivovichev, and V. Depmeier, “Crystal Chemistry of Natural and Synthetic Pb Oxogalogenides. I. Crystalline Structure of Rb13O10Cl6,” Zap. Russian Mineral. O-va 136(4), 79–89 (2007) [Geol. Ore Deposits 136 (Spec. Issue 8, Zapiski Russian Mineral. Soc.), 827–834 (2007)].
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © O.I. Siidra, S.V. Krivovichev, W. Depmeier, 2007, published in Zapiski Rossiiskogo Mineralogicheskogo Obshchestva, 2007, Pt CXXXVI, No. 6, pp. 85–91.
Rights and permissions
About this article
Cite this article
Siidra, O.I., Krivovichev, S.V. & Depmeier, W. Crystal chemistry of natural and synthetic lead oxohalides: II. Crystal structure of Pb7O4(OH)4Br2 . Geol. Ore Deposits 50, 801–805 (2008). https://doi.org/10.1134/S1075701508080187
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1075701508080187