Abstract
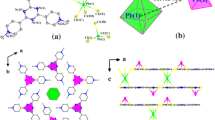
Crystals of lead oxychloride Pb13O10Cl6 have been synthesized on the basis of high-temperature solid-state reactions. The Pb13O10Cl6 structure was studied using X-ray single-crystal diffraction analysis. The compound is monoclinic, and the space group is C2/c; the unit-cell dimensions are a = 16.1699(14), b = 7.0086(6), c = 23.578(2) Å, β = 97.75°, and V = 2647.6(4) Å3. The structure has been solved by direct methods and refined to R 1 = 0.0505 for 2671 observed unique reflections. The structure is a 3D framework consisting of OPb4 tetrahedrons. Chlorine atoms are located in the framework channels. The structure contains seven symmetrically independent Pb atoms, which are coordinated by 2 to 4 O2− and 2 to 4 Cl− anions. The synthesized compound is compared with other natural and synthetic lead oxyhalides.
Similar content being viewed by others
References
N. E. Brese and M. O’Keeffe, “Bond-Valence Parameters for Solids,” Acta Crystallogr. 47, 192–197 (1991).
R. Edwards, R. D. Gillard, P. A. Williams, and A. M. Pollard, “Studies of Secondary Mineral Formation in the PbO-H2O-HCl System,” Mineral. Mag. 56, 53–65 (1992).
O. Gabrielson, “The Crystal Structure of Mendipite, Pb3O2Cl2,” Arkiv. Miner. Geol. 2, 299–304 (1957).
P. Gabrielson, A. Parwel, and F. E. Wickman, “Blixite, a New Lead-Oxyhalide Mineral from Langban,” Arkiv Miner. Geol 2, 411–415 (1958).
D. A. Hirschler, L. F. Gilbert, F. W. Lamb, and L. M. Niebylski, “Particulate Lead Compounds in Automobile Exhaust Gas,” Ind. Eng. Chem. 49, 1131–1142 (1957).
M. Kiyama, K. Murakami, T. Takada, et al., “Formation and Solubility of Basic Lead Chlorides at Different pH Values,” Chem. Lett., 23–28 (1976).
S. V. Krivovichev and I. D. Brown, “Are the Compressive Effects of Encapsulation an Artifact of the Bond Valence Parameters?,” Zschr. Kristallogr. 216, 245–247 (2001).
S. V. Krivovichev and P. C. Burns, “Crystal Chemistry of Lead Oxide Chlorides. I. Crystal Structures of Synthetic Mendipite, Pb3O2Cl2, and Synthetic Damaraite, Pb3O2(OH)Cl,” Eur. J. Mineral. 13, 801–809 (2001).
S. V. Krivovichev and P. C. Burns, “Crystal Chemistry of Lead Oxide Chlorides. II. Crystal Structure of Pb7O4(OH)4Cl2,” Eur. J. Mineral. 14, 135–139 (2002a).
S. V. Krivovicehv and P. C. Burns, “Crystal Structure of Pb10O7(OH)2F2(SO4) and Crystal Chemistry of Lead Oxysulfate Minerals and Inorganic Compounds,” Zschr. Kristallogr. 217, 451–459 (2002b).
S. V. Krivovichev and P. C. Burns, “Chains of Edge-Sharing OPb4 Tetrahedral in the Structure of Pb4O(VO4)2 and in Related Minerals and Inorganic Compounds,” Can. Mineral. 41, (2003).
S. V. Krivovichev and P. C. Burns, “The Crystal Structure of Pb8O5(OH)2Cl4, a Synthetic Analogue of Blixite?,” Can. Mineral. 44, 515–522 (2006).
S. V. Krivovichev and S. K. Filatov, Crystal Chemistry of Minerals and Inorganic Compounds with Complexes of Anion-Centered Tetrahedrons (St. Petersburg State Univ., St. Petersburg, 2001) [in Russian].
S. V. Krivovichev, S. K. Filatov, and T. F. Semenova, “On the Systematics and Description of Polyions of Linked Polyhedra,” Zschr. Kristallogr. 212, 411–417 (1997).
S. V. Krivovichev, O. I. Siidra, E. V. Nazarchuk, et al., “Exceptional Topological Complexity of Lead Oxide Blocks in Pb31O22X18 (X = Br,Cl),” Inorg. Chem. 45, 3846–3848 (2006).
H. Matsumoto, T. Miyake, and H. Iwahara, “Chloride Ion Conduction in PbCl2-PbO System,” Mater. Res. Bull. 36, 1177–1184 (2001).
P. B. Moore, A. R. Kampf, and P. K. Sen Gupta, “The Crystal Structure of Philolithite, a Trellis-Like Open Framework Based on Cubic Closest Packing of Anions,” Am. Mineral. 85, 810–816 (2000).
M. Pasero and D. Vacchiano, “Crystal Structure Refinement of Mendipite, Pb3O2Cl2,” Neues Jahrb. Miner. Mh. 563–569 (2000).
H. J. Riebe and H. L. Keller, “Pn13O10Br6, Ein Neuer Vetreter der Blei(II)-Oxidhalogenide,” Zschr. Anogr. Allg. Chem. 571, 139–147 (1989).
K. Sahl, “Zur Kristalstruktur von Lanarkit, Pb2O(SO4),” Zschr. Kristallogr. 132, 99–117 (1970).
M. B. Sigman, Jr. and B. A. Kirgel, “Strongly Birefringent Pb3O2Cl2 Nanobelts,” J. Am. Chem. Soc. 127, 10089–10095 (2005).
O. I. Siidra, S. V. Krivovichev, and V. Depmaier, “Method of Square Cells As a Method for the Description of Structural Topology of Minerals and Inorganic Compounds Derivative from Tetragonal PbO (Litargite),” Vestn. St. Petersb. Gos. Univ., Ser. 7., No. 3, 18–26 (2006).
W. H. Smith, “Lead Contamination of the Roadside Ecosystem,” J. Air. Pollut. Control. Ass. 26, 753–766 (1976).
L. J. Spencer and E. D. Mountain, “New Lead-Copper Minerals from the Mendip Hills, Somerset, England,” Mineral. Mag. 20, 67–92 (1923).
H. Vincent and G. Perrault, “Structure cristalline de l’oxychlorure de plomb synthétique Pb3O2Cl2,” Bull. Soc. Fr. Minér. Cristallogr. 94, 323–331 (1974).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © O.I. Siidra, S.V. Krivovichev, W. Depmeier, 2007, published in Zapiski Rossiiskogo Mineralogicheskogo Obshchestva, 2007, Pt CXXXVI, No. 2, pp. 79–89.
Rights and permissions
About this article
Cite this article
Siidra, O.I., Krivovichev, S.V. & Depmeier, W. Crystal chemistry of natural and synthetic lead oxyhalides. Part I. Crystal structure of Pb13O10Cl6 . Geol. Ore Deposits 49, 827–834 (2007). https://doi.org/10.1134/S107570150708017X
Received:
Issue Date:
DOI: https://doi.org/10.1134/S107570150708017X