Abstract
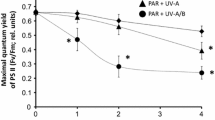
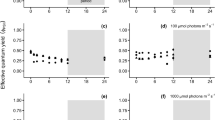
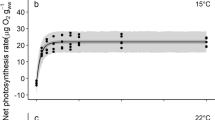
Zoospores of Arctic kelp species, Alaria esculenta, Laminaria digitata and Saccharina latissima were exposed to different temperature (2 °C to 19 °C) and radiation (photosynthetically active radiation (PAR = P), PAR + UV-A (PA), and PAR + UV-A + UV-B (PAB)) conditions in the laboratory. Species-specific responses to the combined effect of light and temperature stress showed sensitivity in the order S. latissima > L. digitata > A. esculenta. The optimum temperature range for photosynthesis in different Arctic kelp species’ zoospores was between 7–13 °C, temperatures higher than in the natural environment. Short-term response to increasing temperature was non-lethal while moderate temperature increase had an ameliorating effect on the overall biological effect of UVR; where the lowest photoinhibition was observed at 13 °C under PAB and higher photosynthetic recovery was observed in UVR-pre-exposed zoospores at 7–13 °C compared to 2 °C. Above the temperature optima, continued cultivation under high temperature had a negative impact on the recovery of photoinhibition. The higher capacity for non-photochemical quenching (NPQ) in A. esculenta and L. digitata helped to regulate and protect photosynthesis under light and temperature stress compared to S. latissima. The investigated Arctic kelp species may be able to locally survive under the influence of UVR at a certain range of temperature increase but the southernmost distribution range of the species may shift to higher latitudes; although natural selection may result in genotypes adapted to stressful environment.
Similar content being viewed by others
References
A. Arguez, A. M. Waple, A. M. Sanchez-Lugo, State of the climate in 2006- executive summary Bull. Am. Meteorol. Soc. 2007 88 929–932.
IPCC, Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, Cambridge, UK, 2007.
F. Joos, R. Spahni, Rates of change in natural and anthropogenic radiative forcing over the past 20,000 years Proc. Natl. Acad. Sci. U. S. A. 2008 105 1425–1430.
M. C. Serreze, J. A. Francis, The Arctic amplification debate Clim. Change 2006 76 241–264.
R. G. Graversen, T. Mauritsen, M. Tjernström, E. Källén, G. Svensson, Vertical structure of recent Arctic warming Nature 2008 541 53–56.
D. K. Perovich, B. Light, H. Eicken, K. F. Jones, K. Runciman, S. V. Nghiem, Increasing solar heating of the Arctic Ocean and adjacent seas, 1979–2005: attribution and role in the ice-albedo feedback Geophys. Res. Lett. 2007 34 L19505 10.1029/2007GL031480.
A. M. Breeman, Expected effects of changing seawater temperatures on the geographic distribution of seaweed species, in Expected Effects of Climatic Change on Marine Coastal Ecosystems, ed. J. J. Beukema, W. J. Wolff and J. W. M. Brouns, 1990, Kluwer Academic Publishers, Netherlands, pp. 69–76.
K. Lüning, in Seaweeds: Their Environment, Biogeography and Ecophysiology, Wiley-Interscience, New York, 1990, 527 pp.
I. tom Dieck, Temperature tolerance and survival in darkness of kelp gametophytes (Laminariales, Phaeophyta): ecological and biogeographical implications Mar. Ecol.: Prog. Ser. 1993 100 253–264.
C. Wiencke, I. Bartsch, B. Bischoff, A. F. Peters, A. M. Breeman, Temperature requirements and biogeography of antarctic, arctic and amphiequatorial seaweeds Bot. Mar. 1994 37 247–259.
D. R. Schiel, J. R. Steinbeck, M. S. Forster, Ten years of induced ocean warming causes comprehensive changes in marine benthic communities Ecology 2004 85 1833–1839.
L. B. Ladah, J. A. Zertuche-González, Survival of microscopic stages of a perennial kelp (Macrocystis pyrifera) from the center and the southern extreme of its range in the Northern Hemisphere after exposure to simulated El Niño stress Mar. Biol. 2007 152 677–686.
P. G. Matson, M. S. Edwards, Effects of ocean temperature on the southern range limits of two understory kelps, Pterygophora californica and Eisenia arborea, at multiple life-stages Mar. Biol. 2007 151 1941–1949.
A. M. Breeman, H. Pakker, Temperature ecotypes in seaweeds: adaptive significance and biogeographic implications Bot. Mar. 1994 37 171–180.
D. W. Fahey, Twenty Questions and Answers About the Ozone Layer: Scientific Assessment of Ozone Depletion: 2002, World Meteorological Organization, Geneva, 2003.
J. J. Jin, K. Semeniuk, G. L. Manney, A. I. Jonsson, S. R. Beagley, J. C. McConnell, G. Dufour, R. Nassar, C. D. Boone, K. A. Walker, P. F. Bernath, C. P. Rinsland, Severe Arctic ozone loss in the winter 2004/2005: observations from ACE-FTS Geophys. Res. Lett. 2006 33 L15801 10.1029/2006GL026752.
G. Muscari, A. G. di Sarra, R. L. de Zafra, F. Lucci, F. Baordo, F. Angelini, G. Fiocco, Middle atmospheric O3, CO, N2O, HNO3, and temperature profiles during the warm Arctic winter 2001–2002 J. Geophys. Res. [Atmos.] 2007 112 D14304 10.1029/2006JD7849.
M. Tedetti, R. Sempéré, Penetration of ultraviolet radiation in the marine environment. A review Photochem. Photobiol. 2006 82 389–397.
D. R. Ort, When there is too much light Plant Physiol. 2001 125 29–32.
A. Melis, Photosystem-II damage and repair cycle in chloroplasts: what modulates the rate of photodamage in vivo? Trends Plant Sci. 1999 4 130–135.
N. Ohnishi, N. S. I. Allakhverdiev, S. Takahashi, S. Higashi, M. Watanabe, Y. Nishiyama, N. Murata, Two-step mechanism of photodamage to photosystem II: step 1 occurs at the oxygen-evolving complex and step 2 occurs at the photochemical reaction center Biochemistry 2005 44 8494–8499.
M. Y. Roleda, D. Hanelt, C. Wiencke, Exposure to ultraviolet radiation delays photosynthetic recovery in Arctic kelp zoospores Photosynth. Res. 2006 88 311–322.
L. R. Davidson, Environmental effects on algal photosynthesis: temperature J. Phycol. 1991 27 2–8.
D. C. Fork, N. Murata, N. Sato, Effect of growth temperature on the lipid and fatty acid composition, and the dependence on temperature of light-induced redox reactions of cytochrome f and of light energy redistribution in the thermophilic blue-green alga Synechococcus lividus Plant Physiol. 1979 63 524–530.
R. Rae, W. F. Vincent, Effects of temperature and UV radiation on microbial food web structure: potential responses to global change Freshwater Biol. 1998 40 1–12.
J. C. Roos, W. F. Vincent, Temperature dependence of UV radiation effects on Antarctic cyanobacteria J. Phycol. 1998 34 118–125.
S. Doyle, J. E. Saros, C. E. Williamson, Interactive effects of temperature and nutrient limitation on the response of alpine phytoplankton growth to UV radiation Limnol. Oceanogr. 2005 50 1362–1367.
C. Sobrino, P. J. Neale, Short-term and long-term effects of temperature on photosynthesis in the diatom. Thalassiosira pseudonana under UVR exposures J. Phycol. 2007 43 426–436.
H. Pakker, C. A. C. Beekman, A. M. Breeman, Efficient photoreactivation of UVBR-induced DNA damage in the sublittoral macroalga Rhodymenia pseudopalmata (Rhodophyta) Eur. J. Phycol. 2000 35 109–114.
H. Pakker, R. S. T. Martins, P. Boelen, A. G. J. Buma, O. Nikaido, A. M. Breeman, Effects of temperature on the photoreactivation of UVB-induced DNA damage in Palmaria palmata (Rhodophyta) J. Phycol. 2000 36 334–341.
W. H. van de Poll, A. Eggert, A. G. J. Buma, A. M. Breeman, Temperature-dependence of UV radiation effects in Arctic and temperate isolates of three red macrophytes Eur. J. Phycol. 2002 37 59–68.
J. R. Hoffman, L. J. Hansen, T. Klinger, Interaction between UV radiation and temperature limit inferences from single-factor experiments J. Phycol. 2003 39 268–272.
R. Müller, C. Wiencke, K. Bischof, Interactive effects of UV radiation and temperature on microstages of Laminariales (Phaeophyceae) from the arctic and North Sea Clim. Res. 2008 37 203–213.
D. Hanelt, H. Tüg, K. Bischof, C. Groß, H. Lippert, T. Sawall, C. Wiencke, Light regime in an arctic fjord: a study related to stratospheric ozone depletion as a basis for determination of UV effects on algal growth Mar. Biol. 2001 138 649–658.
A. D. Jassby, T. Platt, Mathematical formulation of the relationship between photosynthesis and light for phytoplankton Limnol. Oceanogr. 1976 21 540–547.
I. tom Dieck, North Pacific and North Atlantic digitate Laminaria species (Phaeophyta): hybridization experiments and temperature responses Phycologia 1992 31 147–163.
C. Wiencke, M. N. Clayton, M. E. A. Schoenwaelder, Sensitivity and acclimation to UV radiation of zoospores from five species of Laminariales from the Arctic Mar. Biol. 2004 145 31–39.
A. R. O. Chapman, J. E. Lindley, Seasonal growth of Laminaria solidungula in the Canadian High Arctic in relation to irradiance and dissolved nutrient concentrations Mar. Biol. 1980 57 1–5.
W. H. Adey, S. C. Lindstrom, M. H. Hommersand, K. M. Müller, The biogeographic origin of Arctic endemic seaweeds: a thermogeographic view J. Phycol. 2008 44 1384–1394.
E. C. Henry, Primitive reproductive characters and a photoperiodic response in Saccorhiza dermatodea (Laminariales, Phaeophyceae) Br. Phycol. J. 1987 22 23–31.
E. C. Henry, Regulation of reproduction in brown algae by light and temperature Bot. Mar. 1988 31 353–357.
I. R. Davison, Adaptation of photosynthesis in Laminaria saccharina (Phaeophyta) to changes in growth temperature J. Phycol. 1987 23 273–283.
I. R. Davison, R. M. Greene, E. J. Podolak, Temperature acclimation of respiration and photosynthesis in the brown alga Laminaria saccharina Mar. Biol. 1991 110 449–454.
K. M. Machalek, I. R. Davison, P. G. Falkowski, Thermal acclimation and photoacclimation of photosynthesis in the brown alga Laminaria saccharina Plant Cell Environ. 1996 19 1005–1016.
N. P. A. Huner, G. Öquist, F. Sarhan, Energy balance and acclimation to light and cold Trends Plant Sci. 1998 3 224–230.
I. Ensminger, F. Busch, N. P. A. Huner, Photostasis and cold acclimation: sensing low temperature through photosynthesis Physiol. Plant. 2006 126 28–44.
J. Bruhn, V. A. Gerard, Photoinhibition and recovery of the kelp Laminaria saccharina at optimal and superoptimal temperatures Mar. Biol. 1996 125 639–648.
T. D. Sharkey, Effects of moderate heat stress on photosynthesis: importance of thylakoid reactions, rubisco deactivation, reactive oxygen species, and thermotolerance provided by isoprene Plant Cell Environ. 2005 28 269–277.
N. Suzuki, R. Mittler, Reactive oxygen species and temperature stresses: a delicate balance between signaling and destruction, Physiol. Plant. 126 45-51.
M. Y. Roleda, C. Wiencke, D. Hanelt, W. H. van de Poll, A. Gruber, Sensitivity of Laminariales zoospores from Helgoland to ultraviolet and photosynthetically active radiation: implication for depth distribution and seasonal reproduction Plant Cell Environ. 2005 28 466–479.
V. A. Gerard, K. DuBois, R. Greene, Growth responses of two Laminaria saccharina populations to environmental variation Hydrobiologia 1987 151/152 229–232.
B. de Reviers, F. Rousseau, Towards a new classification of the brown algae Prog. Phycol. Res. 1999 13 107–201.
P. A. Wilson, R. D. Norris, Warm tropical ocean surface and global anoxia during the mid-Cretaceous period Nature 2001 412 425–429.
J. Zachos, M. Pagani, L. Sloan, E. Thomas, K. Billups, Trends, rhythms, and aberrations in global climate 65 Ma to present Science 2001 292 667–671.
J. A. Raven, A. M. Johnston, J. E. Kübler, R. Korb, S. G. McInroy, L. L. Handley, C. M. Scrimgeour, D. I. Walker, J. Beardall, M. N. Clayton, M. Vanderklift, S. Fredriksen, K. H. Dunton, Seaweeds in cold seas: evolution and carbon acquisition Ann. Bot. 2002 90 525–536.
C. E. Lane, C. Mayes, L. D. Druehl, G. W. Saunders, A multigene molecular investigation of the kelp (Laminariales, Phaeophyceae) supports substantial taxonomic reorganization J. Phycol. 2006 42 493–512.
A. G. B. Poore, T. Fagerström, Intraclonal variation in macroalgae: causes and evolutionary consequences Selection 2000 1–3 123–133.
V. D. Kreslavski, R. Carpentier, V. V. Klimov, N. Murata, S. I. Allakhverdiev, Molecular mechanisms of stress resistance of the photosynthetic apparatus Biol. Membr. 2007 24 195–217.
D. Hanelt, M. Y. Roleda, UVB radiation may ameliorate photoinhibition in specific shallow-water tropical marine macrophytes Aquat. Bot. 2009 10.1016/j.aquabot.2008.12.005.
L. W. Jones, B. Kok, Photoinhibition of chloroplast reactions. I. Kinetics, and action spectra Plant Physiol. 1966 41 1037–1043.
J. Grzymski, C. Orrico, O. M. Schofield, Monochromatic ultraviolet light induced damage to Photosystem II efficiency and carbon fixation in the marine diatom Thalassiosira pseudonana (3H) Photosynth. Res. 2001 68 181–192.
E. Turcsányi, I. Vass, Effect of UV-A radiation on photosynthetic electron transport Acta Biol. (Szeged) 2002 46 171–173.
M. Richter, W. Rühle, A. Wild, Studies on the mechanism of Photosystem II photoinhibition I. A two-step degradation of D1-protein Photosynth. Res. 1990 24 229–235.
D. P. Häder, F. L. Figueroa, Photoecophysiology of marine macroalgae Photochem. Photobiol. 1997 66 1–14.
E. M. Purcell, Life at low Reynolds number Am. J. Phys. 1977 45 3–11.
M. Y. Roleda, M. N. Clayton, C. Wiencke, Screening capacity of UV-absorbing compounds in spores of Arctic Laminariales J. Exp. Mar. Biol. Ecol. 2006 338 123–133.
C. Wiencke, M. Y. Roleda, A. Gruber, M. N. Clayton, K. Bischof, Susceptibility of zoospores to UV radiation determines upper depth distribution limit of Arctic kelps: evidence through field experiments J. Ecol. 2006 94 455–463.
P. Müller, X.-P. Li, K. K. Niyogi, Non-photochemical quenching. A response to excess light energy Plant Physiol. 2001 125 1558–1566.
I. Szabó, E. Bergantino, G. M. Giacometti, Light and oxygenic photosynthesis: energy dissipation as a protection mechanism against photo-oxidation EMBO Rep. 2005 6 629–634.
J. D. Robison, M. E. Warner, Differential impacts of photoacclimation and thermal stress on the photobiology of four different phylotypes of Symbiodinium (Pyrrhophyta) J. Phycol. 2006 42 568–579.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article was published as part of the themed issue on “Environmental effects of UV radiation”
Rights and permissions
About this article
Cite this article
Roleda, M.Y. Photosynthetic response of Arctic kelp zoospores exposed to radiation and thermal stress. Photochem Photobiol Sci 8, 1302–1312 (2009). https://doi.org/10.1039/b901098j
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/b901098j