Abstract
Whole-genome sequencing methods in familial cancer are useful to unravel rare clinically important cancer predisposing variants. Here, we present improvements in our pedigree-based familial cancer variant prioritization pipeline referred as FCVPPv2, including 12 tools for evaluating deleteriousness and 5 intolerance scores for missense variants. This pipeline is also capable of assessing non-coding regions by combining FANTOM5 data with sets of tools like Bedtools, ChromHMM, Miranda, SNPnexus and Targetscan. We tested this pipeline in a family with history of a papillary thyroid cancer. Only one variant causing an amino acid change G573R (dbSNP ID rs145736623, NM_019609.4:exon11:c.G1717A:p.G573R) in the carboxypeptidase gene CPXM1 survived our pipeline. This variant is located in a highly conserved region across vertebrates in the peptidase_M14 domain (Pfam ID PF00246). The CPXM1 gene may be involved in adipogenesis and extracellular matrix remodelling and it has been suggested to be a tumour suppressor in breast cancer. However, the presence of the variant in the ExAC database suggests it to be a rare polymorphism or a low-penetrance risk allele. Overall, our pipeline is a comprehensive approach for prediction of predisposing variants for high-risk cancer families, for which a functional characterization is a crucial step to confirm their role in cancer predisposition.
Similar content being viewed by others
Introduction
Oncogenomics has been boosted with rapid advancements in the next-generation sequencing (NGS) technologies in the last 10 years with large consortia describing several thousands of somatic variants. However, there has been far less success in the discovery of new cancer predisposing genes (CPGs) as only some new genes were identified using germline genome sequencing1. The major fraction of CPGs were discovered in the late 1990s using the familial linkage analysis1. Since then the interest in collecting cancer pedigrees diminished and the consequence has been that whole exome/genome sequencing (WES/WGS)-based family studies have been forced to resort either to small pedigrees or single cases from affected families2. In cancer studies both types of approaches have been used3,4,5,6. CPGs found include for example an NTHL1 variant in colorectal cancer3 and an RECQL variant in breast cancer4.
As pedigree-based studies have a high discriminatory power if samples from many affected and unaffected members are available, we recently introduced the familial cancer variant prioritization pipeline (FCVPP5), which is a pipeline capable of detecting rare germline variants and their corresponding CPGs. In the current study, we describe FCVPPv2, an upgraded version of our FCVPP pipeline5. FCVPPv2 prioritizes rare deleterious and regulatory germline variants, both in the coding and non-coding region for cancer families. The advantages of this approach are several-fold such as (a) reducing the large number of variants through the pedigree segregation step; (b) assessing the deleterious nature of missense variants by a combination of 12 ranking tools and 5 intolerance scores; (c) analysis of non-coding variants by specialized tools such as Miranda7 and Targetscan 7.08 for 3′ UTR variants, SNPnexus9 for 5′ UTR variants and FANTOM5 and SNPnexus9 for variants in enhancers10 and promoters11,12 and (d) this pipeline also takes advantages of the improvements of population frequencies in public databases, which assists inferring rarity of a particular variant. Herein, we present the improvements of FCVPPv2 as well as its application to a pedigree of papillary thyroid cancer (PTC).
Results
Familial cancer variant prioritization pipeline version 2 (FCVPPv2) comes with several improvements
Overview of FCVPPv2 is provided in Figs 1 and 2 and description of this work involves several abbreviations of scientific words and tools as summarized in Supplementary Table S1. FCVPPv2 has implemented platypus tool13 for joint variant calling. It combines several resources for variant annotation like ANNOVAR14, exome aggregation consortium (ExAC15), exonic variant server with 6500 samples (EVS650016), 1000 Genomes phase III17, dbNSFP v3.018, and dbSNP19.
Summary of familial cancer variant prioritization pipeline version 2 (FCVPPv2). This pipeline uses platypus tool13 for joint variant calling after mapping of the sequencing reads from cases and controls. FCVPPv2 uses several external tools for variant annotation namely ExAC, 1000 Genomes phase III data, ANNOVAR and dbNSFPv3, dbSNP and EVS6500. For candidate variants the variants are filtered using read quality parameters like coverage and quality scores (QUAL) must be >5 and >20, respectively. Minor allele frequency (MAF) must be below 0.1% in the European populations in all used databases. Furthermore, these variants are screened with respective to family-pedigree and this is the most critical step in the germline genomics (shown in black shade). After this step, variants are ranked with the help of CADD v1.320 and any variants with CADD PHRED score of >10 belongs to top 10% for probable functional and deleterious variants in the human genome. These deleterious variants are subsequently divided into 4 different categories based on their locations. The coding variants are considered deleterious based on the consensus from 12 deleteriousness prediction tools and 5 intolerance scores. Variants in the 5′ UTR are considered regulatory based on the Haploreg V4.121, RegulomeDB22 and SNPnexus9 while variants in the 3′ UTR are regulatory if supported by the presence of miRNA binding site using Miranda7 and Targetscan 7.08 tools and additional hints are received from Haploreg V4.121 and RegulomeDB22. For variants in the non-coding segments we combined several state-of- the-art tools such as chromHMM, Segway, FunSeq2 and FANTOM5 data. Non-coding (intergenic and intronic) variants may not always have CADD > 10 even though they will have regulatory implications, so we analyzed all non-coding variants after pedigree segmentation, either with or without CADD > 10. Putative deleterious or regulatory variants are visualized using Locuszoom, SniPA and UCSC genome browser. Potential variants are also checked with sets of additional features, e.g. list of known CPGs1 and clinically relevant variants (ClinVar), expression data and somatic mutations. We also checked the sequencing data of all cases and controls in a particular family for correctness using the IGV browser.
Overview of strategies for regulatory variant detection in the non-coding segments of the human genome. We utilized the FANTOM5 data using the SlideBase Tool (slidebase.binf.ku.dk) with 32693 enhancers and 184476 promoters (downloaded in March 2017). We matched our variants (pedigree segregated) with FANTOM5 data using Bedtools intersect to retrieve a list of potentially critical variants localized within promoters and/or enhancers, and we examined the status of transcription factor (TF) binding sites using SNPnexus9. We checked the signals for chromatin binding using ChromHMM and genomic segmentation data from Segway via CADDv1.320. In addition, we examined if the putative noncoding regulatory variants were localized in the ultra-conserved non-coding elements (UCNEs) and their clusters, also known as ultra-conserved genomic regulatory blocks (UGRBs) with the help of UCNEbase24 and also if these variants were located in ultra-sensitive and sensitive regions (Ultrasen), defined by FunSeq223. The top-ranked variants were examined for their regulatory nature by using Locuszoom, SniPA, UCSC and ZENBU genome browsers. We also examined if the putative enhancer variant fall into the category of super-enhancers using super-enhancer archive (SEA)25 and dbSUPER26. Expression profile, RNA-seq data-based information and motif changes and disruptions were gathered with help from FANTOM5 data via the SlideBase.
Variants are filtered with criteria using read quality parameters like coverage and quality scores (QUAL) must be >5 and >20, respectively. FCVPPv2 uses minor allele frequency (MAF) below 0.1% in the European populations in all used databases. The hallmark of FCVPPv2 is use of family-pedigree variant screening (Fig. 1). Post this step, CADD v1.320 based variant filtering is performed with PHRED CADD score of 10 as a cut-off. Subsequently, deleterious variants are segregated on their locations. The coding variants are considered deleterious based on the consensus from 5 intolerance scores, 3 conservational scores and 12 deleteriousness prediction tools (Fig. 1, Tables 1 and 2). FCVPPv2 characterizes the regulatory nature in the UTR regions by combining a set of tools like the Haploreg V4.121, RegulomeDB22 and SNPnexus9 for variants in 5′ UTR and miRNA binding sites using Miranda7 and Targetscan 7.08 tools for variants in 3′ UTRs (Fig. 1).
Above 98% of the human genome is non-coding and dealing with non-coding variants is a daunting task. No tools can accurately predict the regulatory nature of non-coding variants. To overcome this issue, FCVPPv2 uses a combination of the several standard tools like chromHMM, Segway, FunSeq223 and FANTOM5 data (Fig. 2). FCVPPv2 focuses the FANTOM5 data by matching variants within promoters and/or enhancers using Bedtools intersect function. FCVPPv2 makes assessments of transcription factor (TF) binding sites using SNPnexus9. This pipeline makes use of signals for chromatin binding using ChromHMM and genomic segmentation data from Segway via CADDv1.3. Additionally, FCVPPv2 checks if a putative non-coding regulatory variant is localized in the ultra-conserved non-coding elements (UCNEs) or ultra-conserved genomic regulatory blocks (UGRBs) with the help of UCNEbase24, and in ultra-sensitive and sensitive regions (Ultrasen), defined by FunSeq223. FCVPPv2 uses Bedtools intersect function to assign variants in regions of super-enhancers using super-enhancer archive (SEA)25 and dbSUPER26 databases.
FCVPPv2 visualises top ranked variants for their regulatory natures using different genome browsers like Locuszoom27, SNiPA28, the UCSC29 and ZENBU30 (Fig. 1). The status of RNA and protein expression for genes carrying potential deleterious variants is examined with the help of FANTOM5, BioGPS31 and Human Protein Atlas32 (Fig. 1). This tool uses literature mining to check if these variants are found in known lists of cancer predisposing genes (CPGs1). Towards end, FCVPPv2 provides a summary of potential variants by combining features from several databases like ClinVar33, Online Mendelian Inheritance in Man (OMIM, https://omim.org/), CanVar Browser34, cBioPortal35, COSMIC36, ICGC37 and IntOGen38 (Fig. 1).
Application of FCVPPv2 to a papillary thyroid cancer (PTC) family
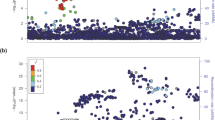
We used FCVPPv2 to a family with PTC with two distantly related cases (sample no. 2 and 3) and one unrelated case (sample no. 1, Fig. 3A). We ranked top variants for this family after WGS of the three samples. After variant annotation and removal of variants with MAF higher than 0.1% in at least one variant database, a total of 120,323 variants remained (Fig. 3B & Supplementary Table S2). We applied pedigree-filtering criteria imposing that the two related cases must have the variant, while the unrelated case should not have it. With this pedigree filtering approach we narrowed down the potential list of variants to 1970, which is about 1.6% of the initial variant set (Fig. 3B). Subsequently, we examined these variants based on their location in the genome and we found that only 28 of them were located in the coding region, which is 1.4% of all pedigree segregated variants, while 98.6% of them were located in the non-coding region with 1015 intronic, 901 intergenic and 26 up- or down-stream variants (Fig. 3B). Out of the 28 coding variants only 7 had a CADD PHRED score >10 and these included 5 exonic (4 non-synonymous and 1 non-frameshift insertion) and two UTR (1 in 3′ UTR and 1 in ncRNA_UTR) variants (Fig. 3B and Table 3). The non-frameshift insertion and the UTR variants (Table 3) were indels localized in the repetitive regions in the human genome and hence these were not considered further. Of the non-coding variants, none was located within a predicted enhancer or promoter.
We focused on the 4 non-synonymous variants localized in four different genes-C1orf27 (chromosome 1 open reading frame 27), CPXM1 (carboxypeptidase X (M14 family), member 1), FAM129A (family with sequence similarity 129, member A) and ZBTB41 (zinc finger and BTB domain containing 41) (Figs 3B and 4A). All these 4 variants had CADD score >20, which indicates top 1% deleterious variants in the human genome. The highest CADD score of 32 was reported for CPXM1 ranking it to the list of top 0.1% deleterious variants. We prioritized the variant G573R in CPXM1 as our top candidate, as it had very high conservation scores (GERP++ = 5.3, phastCons = 1.0, and phyloP = 7.6), and 9 out of the 12 deleterious prediction scores and 4 out of the 5 intolerance scores were favoring it. Also amino acid change from glycine to arginine is critical with a Grantham score of 125 (moderately radical, deduced from CADD annotation20), while the remaining three variants had a low Grantham score (<30). Additionally, although the three other variants had a CADD score >20, mutations in C1orf27 and FAM129A were classified as tolerated by all 5 intolerance scoring tools (listed in Table 1) and the ZBTB41 variant was only predicted to be deleterious by 3 out of 12 tools (Fig. 4A). All in all, we found one predicted deleterious missense variant in the CPXM1 gene, which was not found in any other 77 cancer families (including 4 PTC families), we have whole-genome sequenced by now. We only found two other missense variants in the entire CPXM1 gene in three different families out of the 77 cancer families, each present either in only one case or only one control of the family (Supplementary Table S3). During the course of this study, the NM_019609.4:exon11:c.G1717A:p.G573R variant was identified in one colorectal cancer patient (Supplementary Table S4) as reported in the CanVar Browser34 (a database of genetic variants of 1,006 early-onset familial colorectal cancer cases6). We also found a rare stop gained variant and 3 more frequent missense variants in this gene in the CanVar Browser34 (Supplementary Table S4). Currently, the G573R variant (dbSNP ID - rs145736623) is also listed in the ExAC database with a frequency of 0.0004 in the total population (0.0006 in the European, non-Finnish population). Taken together, as we found the NM_019609.4:exon11:c.G1717A:p.G573R variant in 2 distantly related cases of our PTC family, it may be a low-penetrance allele predisposing to PTC, but it may also be a rare polymorphism.
Overview of the top missense variants in the PTC family. (A) The 4 top ranked variants are shown with their favorable and unfavorable features. Grantham scores - 0–50 - conservative, 51–100 - moderately conservative, 101–150 - moderately radical and ≥151 - radical. (B) Location of the G573R variant in the peptidase M14 domain of CXPM1. (C) The G573R variant is localized in a highly conserved region. CXPM1 protein sequences were downloaded from GenBank as human (GenBank ID - NP_062555.1), gorilla (XP_004061758.1), cat (XP_003983774.1), pig (XP_003134381.1), seal (XP_021544821.1), lizard (XP_008120663.1), Xenopus (XP_002936314.1), catfish (XP_017320329.1), carp (XP_018934262.1), molly (XP_014844715.1) and zebrafish (XP_693256.4).
Characterization of the CPXM1 variant and potential roles of the CPXM1 gene
Our familiar PTC candidate variant (NM_019609.4:exon11:c.G1717A:p.G573R) is located at the end of exon 11 of the CPXM1 gene, which is composed of 14 exons (Fig. S1A). To confirm the accuracy of variant calling, we examined the genomic data of all sequenced samples using Integrative Genomics Viewer (IGV)39, reassuring that this CPXM1 variant is only present in the two related cases (sample no. 2 and 3, Fig. S1B), but not in the unrelated case (sample no. 1).
The human CPXM1 gene (also known as CPX1) encodes a zinc metallocarboxypeptidase. The human CPXM1 protein is 734 amino acids long (Fig. 4B). Upon Pfam domain scanning, we found that the CPXM1 protein is composed of three Pfam domains, namely F5_F8_type_C (PFam ID - PF00754), peptidase_M14 (PF00246) and carboxypepD_reg (PF13620) located at amino acid positions 132–271, 306–611 and 625–688, respectively. The CPMX1 variant (NM_019609.4:exon11:c.G1717A:p.G573R) is localized in the peptidase_M14 domain (Fig. 4B). Upon examination the 25 amino acids flanking the variant position, we found the G573 residue and the majority of the flanking residues to be highly conserved from human to zebrafish (Fig. 4C). The CPXM1 protein has no carboxypeptidase activity but it is a secreted N-glycoprotein that binds collagen40. It has been reported to be involved in adipogenesis through extracellular matrix remodeling41. Peroxisome proliferator-activated receptor gamma (PPARγ) is the master adipogenic regulator and it may promote growth and invasion of undifferentiated thyroid cancer (TC) cells42. Whether CPXM1 may predispose to differentiated TC by acting as a complementary regulator to PPARγ in adipogenesis or through extracellular matrix remodeling, remains to be discovered41. There is experimental evidence indicating that expression of CPXM1 is epigenetically regulated in breast cancer and it may act as a tumor suppressor gene43,44. Recently, an indirect role for CPXM1 in PTC was also illustrated, as CPXM1 was downregulated by a long non-coding RNA (lncRNA, Ensembl ID - ENSG00000273132.1)45.
Discussion
The main theme for understanding the germline cancer genetics is the identification of pathogenic mutations and genes predisposing to cancer. Rapid improvements in scientific and technological aspects of genomics have contributed to revolutionary changes in cancer genetics in particularly in cancer treatment but also in cancer risk assessment, cancer screening and prevention, thus setting up a milestone for approaching towards personalized medicine46. With these advancements and decreasing costs, WES/WGS has become the state-of-the-art tool for identifying susceptibility loci in several types of Mendelian diseases2. There are a handful of successful reports on disease gene identification for cancer syndromes such as TERT promoter mutation47 and POT1 mutations48 in familial melanoma, POLE, POLD149 and FAN150 mutations in familial colorectal cancer and KDR mutation51 in familial Hodgkin lymphoma. However, delivering one out of several millions of human genetic variants as the main cause of hereditary cancer is a daunting computational task2. Recently, we developed a pipeline for this purpose, which is known as the FCVPP5. We applied several types of improvements to this pipeline while working with different cancer families and now we are representing the second version of this pipeline as FCVPPv2. FCVPPv2 can deal with missense variants in a more sophisticated way by employing 12 deleteriousness assessment tools and 5 intolerance scores. Additionally, it has the capability of dealing with non-coding variants by the use of data from FANTOM5, super-enhancer databases, UCNEbase and FunSeq2, and without CADD filter as CADD may not pick up many non-coding positions as deleterious.
As an example we showed our experience in hunting predisposing genes for PTC in a high risk PTC family. Our approach involved WGS of germline DNA from several affected and unaffected family members. As a consequence, we found that only 1 out of 61 variants (1970 variants out of 120,323 rare variants remained after pedigree segregation) was able to pass the pedigree segregation filter (Fig. 3B). Hence, pedigree-based variant filtering is a highly effective way for filtering out non-causative variants; in this case only <1.6% of the rare variants survived this step.
We identified 28 variants out of 1970 (1.4%) as coding region variants, which is close to the proportion of coding regions of the whole genome (2%). A single coding variant, CPMX1 (NM_019609.4:exon11:c.G1717A:p.G573R), passed our filtering criteria, CADD score >10, location in a highly conserved region, deleterious in >60% of the prediction tools and intolerance tools predicting it to be deleterious. We had three indel variants in the coding and untranslated regions but they were in repetitive genomic segments and hence were not considered for final prioritization. Furthermore, none of the 1942 non-coding variants were predicted to be located within a promoter or an enhancer. Literature search gave some evidence about a potential function of the CPXM1 gene in cancer. CPXM1 may serve as a tumour suppressor in breast cancer43,44, potentially through involvement in adipogenesis or extracellular matrix remodelling40,41, and it is reported to be down-regulated by a lncRNA in PTC45. Our study identified one predicted pathogenic mutation located in the peptidase M14 domain of the CPMX1 protein in the PTC family, yet this domain is inactive in CPMX1 protein and lacks a typical carboxypeptidase function. Recent sequencing data on colorectal cancer and ExAC databases suggest that the identified variant may be a rare polymorphism. Alternatively it may be a low-penetrance PTC predisposing variant, as it was found in two distant relatives, with no other known PTCs in the family. Lack of functional data of the CPXM1 gene complicates further evaluation of its function and the pathways it is involved. As tumour samples from the mutation carriers of the PTC family are not available, we cannot explore the tumour suppressor nature of CPMX1 in PTC.
In general, our understanding of human genes and their roles in human diseases, including cancer, are still limited. Lack of proper annotation and unknown physiology hampers mechanistic groundwork for candidate variants. Genes involved in known pathways and with more information in the literature are more likely to be studied further than genes with little or no functional characterization. Without this knowledge or convincing segregation data there may be doubts to accept the detected genes as new bona fide tumor suppressor genes. Recent findings on HABP2 gene in nonmedullary thyroid cancer52, deubiquitinating enzyme coding BAP1 in multiple cancers53, ovarian cancer gene 1 (OVCA1)54, promyelocytic leukemia protein (PML)55 and regucalcin (RGN)56 are offered by variable levels of supporting functional evidence. From these, the HABP2 mutation52 was later shown to be a common polymorphism57. Another problem faced by the WES/WGS results in Mendelian diseases is also highlighted in our study: the potentially pathogenic mutation was found only in one family2.
An estimate from 2012 suggested that WES/WGS studies in Mendelian diseases have a success rate of about 60–80%2. However in cancer only handful of novel CPGs were found through WGS/WES58. We think that cancer is more complex than other Mendelian diseases because carcinogenesis is the interplay of germline and somatic events in the form of tumour growth.
In this study, we provide details of a variant prioritization pipeline FCVPPv2 for gene identification in high-risk cancer families applying pedigree segregation-based variant filtering and variant prioritization using state-of-the-art bioinformatics tools and databases. This pipeline detected a predicted deleterious variant in the CPMX1 gene in a PTC family. However, as recent sequencing efforts have found the variant in both the ExAC population and a colorectal cancer family member, experimental validation of the identified variant and functional characterization of the gene are necessary for mechanistic understanding and evaluation of the potential cancer predisposing nature of CPMX1.
Material and Methods
Ethic permissions for the experiments from two committees
All experimental protocols were approved by two ethical committees namely “Comitato Etico Indipendente dell ‘Azienda Ospedaliero-Universitaria di Bologna, Policlinico S. Orsola-Malpighi (Bologna, Italy)” and “comité consultatif de protection des personnes dans la recherche biomédicale, Le centre de lutte contre le cancer Léon-Bérard (Lyon, France)”. Sample collection was carried out in accordance with relevant guidelines and regulations of these two committees. As per guidelines of these two committees, we obtained informed consents of patients for use of their blood samples and the DNA extraction from bloods.
Whole-exome/genome sequencing, mapping, variant calling, filtering and annotation
WES/WGS of the cases and controls from different families considered into the current study was performed in the Illumina X10 platform using DNA extracted from the blood samples. WES/WGS was performed as a paired-end sequencing with a read length of 150 bp. Mapping of reads to reference human genome (assembly version hs37d5) was performed using BWA-0.7.8.r2.05 mem (convey and alignment parameter as –T 0)59 and duplicates were removed using bammarkduplicates-0–0.148. Variants were called by using Platypus-0.8.113 (with details as Platypus-0.8.1.py callVariants —genIndels = 1 —genSNPs = 1 —minFlank = 0). Variants were annotated using ANNOVAR14, dbNSFP v3.018, 1000 Genomes phase III17, dbSNP19 and ExAC15. Variant filtering was performed with considering the quality score >20 and coverage of minimum 5 reads. Minor allele frequency (MAF) of 0.1% was used with respect to population databases (the 1000 Genomes phase III17, non-TCGA exome aggregation (ExAC) data15, and local data sets). A pairwise comparison of variants among the cohort was performed to check for sample swaps and family relatedness.
Segregation in Pedigrees
The variants were filtered separately in each family based on the pedigree data by considering cancer patients as cases and unaffected persons as controls, and by applying to each individual a probability consideration for being a Mendelian case or a true control. However, a rule of thumb was that a maximum number of cases and a minimum number of controls in each family must carry the variant.
Variant ranking using combined annotation dependent depletion (CADD)
After pedigree segregation, variants were prioritized using the CADD tool v1.320 with the scaled PHRED-like CADD score greater than 10, which accounts for top 10% of probable deleterious variants in the human genome. Similarly the scores >20 and >30 are indicative of the top 1% and top 0.1% of deleterious variants, respectively20. All variants with CADD score >10 were taken into further consideration.
Screening genic variants using intolerance score
Intolerance score ranks genes based on their capabilities to possess more or less common functional genetic variation relative to the genome wide expectation. Hence, genes, which have less common functional variation, are referred to as “intolerant” genes while genes prone to have more variations are called as “tolerant” genes. Tools were developed for calculating intolerance scores as reported in Table 1. Initially, residual variation intolerance score (RVIS) was developed based on allele frequency data as represented in whole exome sequence data from the NHLBI-ESP6500 data set60. We used this scoring system with ExAC15 and with our local datasets to create two additional intolerance scores, which were included into the prioritization process (Table 1).
Based on large-scale exome sequencing data, the ExAC consortium has developed two scoring systems which are known as loss-of-function (LoF) intolerance score (pLI score) and Z-score for missense and synonymous variants15. The pLI score is the probability that a gene is intolerant to a LoF mutation. There are three major classes for LoF mutations: (i) null, where LoF variation is completely tolerated; (ii) recessive, where heterozygous LoFs are tolerated; and (iii) haploinsufficient, where heterozygous LoFs are not tolerated. The closer the pLI score is to 1, the less tolerant the gene is to LoF, with pLI > = 0.9 reflecting an extremely LoF-intolerant set of genes. Similarly, the Z-score was developed by the ExAC consortium for missense and synonymous variants. The Z-score is based on the deviation of the observed from the expected number (Table 1). Positive Z-scores indicate that the gene has less variants than expected and hence is intolerant to variation, while genes with more variants will have negative Z scores15.
Conservational screening of variants
Since high evolutionary conservation indicates functional importance of a position, it can be used to predict if a variant is deleterious or not. Based on this approach, evolutionary conservation-based parameters were developed as summarized in Table 1. Genomic Evolutionary Rate Profiling (GERP)61 and the PhastCons62 were utilized for the assessments of variant conservation with the GERP score of >2.0 and the PhastCons score of >0.3 indicating a high level of conservation of the variant position and were used as threshold in the screening of variants. PhyloP is a module of the PHAST package63, which calculates p-values for conservation using a defined multiple alignment63. PhyloP scores range from −14 to + 6 where a higher score indicates a higher level of conservation. During the variant ranking process, a PhyloP score ≥3.0 was used as a criterion for a high level of conservation (Table 1).
Screening missense variants using 12 deleterious ranking tools
All missense variants were assessed for deleteriousness using 12 tools as summarized in Table 2. These tools were developed using information based on (a) sequence conservation, (b) structure, (c) combination of sequence and structure information and (d) meta-prediction using already known tools. SIFT is based on sequence data64, while PolyPhen uses both sequence and structure features65, and MetaSVM and MetaLR are combining pre-existing tools and hence these are examples of meta prediction tools18. Scores from these tools were gathered using dbNSFP18. Variants predicted to be deleterious by at least 60% of these tools were analysed further.
Prediction of regulatory nature of the non-coding variants
Putative miRNA targets among the 3′ UTR variants were detected using the miRanda suite; a mirSVR score lower than −0.1 is indicative of a “good” miRNA target7. Furthermore, we used entire dataset of the human miRNA target atlas from targetscan 7.08 and scanned it with help of the intersect function of the bedtools66,67. The 5′ UTRs were scanned for transcription factor binding sites using SNPnexus9. For regulatory variants, we merged enhancer10 and promoter11,12 data from the FANTOM5 consortium using the intersect function of bedtools. We employed a similar strategy for variants potentially localized in the super enhancer regions using super-enhancer archive (SEA)25 and dbSUPER26. Furthermore, the regulatory nature and impact of non-coding variants were assessed using CADD v1.320, HaploReg V421 and RegulomeDB22, which are based mainly on the ENCODE data68. SNPnexus9 was used to evaluate changes in transcription factor binding sites. Additionally, epigenomic data and marks from 127 cell lines from the NIH Roadmap Epigenomics Mapping Consortium were accessed via CADD v1.320 for regulatory variants. We also tested if our variants were located within the ultra-conserved non-coding elements (UCNEs) and their clusters also known as ultra-conserved genomic regulatory blocks (UGRBs) with the help of UCNEbase24 and also if these variants were located in ultra-sensitive regions (Ultrasen), defined by FunSeq223.
Visualization of the variants
Variants were visualized in the human genome (version hg19) using the Locuszoom27, SNiPA28, the UCSC29 and ZENBU30 genome browsers.
Ranked deleterious variants were examined for additional features
Potential variants were examined carefully for several additional features like if these variants were found in known list of CPGs1. It was also examined whether clinical data and associated phenotypic data from ClinVar33, Online Mendelian Inheritance in Man (OMIM, https://omim.org/) and other disease gene databases were available for the concerned variants. The status of RNA and protein expression for genes carrying potential deleterious variants was examined with the help of BioGPS31 and Human Protein Atlas32, respectively. We also checked if the concerned germline variant was already reported in known germline variant databases like CanVar Browser34 or in the somatic mutation cancer databases like cBioPortal35, COSMIC36, ICGC37 and IntOGen38. The sequencing data for the concerned variants were rechecked manually using Integrative Genomics Viewer (IGV)39 and validated using Sanger sequencing.
Other data analysis by FCVPPv2
We can assist with data analysis using FCVPPv2, please send us a personal communication to either AK (a.kumar@dkfz.de) or AF (a.foersti@dkfz.de).
References
Rahman, N. Realizing the promise of cancer predisposition genes. Nature 505, 302–308 (2014).
Gilissen, C., Hoischen, A., Brunner, H. G. & Veltman, J. A. Disease gene identification strategies for exome sequencing. European journal of human genetics: EJHG 20, 490–497, https://doi.org/10.1038/ejhg.2011.258 (2012).
Weren, R. D. et al. A germline homozygous mutation in the base-excision repair gene NTHL1 causes adenomatous polyposis and colorectal cancer. Nat Genet 47, 668–671, https://doi.org/10.1038/ng.3287 (2015).
Cybulski, C. et al. Germline RECQL mutations are associated with breast cancer susceptibility. Nat Genet 47, 643–646, https://doi.org/10.1038/ng.3284 (2015).
Forsti, A. et al. Pedigree based DNA sequencing pipeline for germline genomes of cancer families. Hereditary cancer in clinical practice 14, 16, https://doi.org/10.1186/s13053-016-0058-1 (2016).
Chubb, D. et al. Rare disruptive mutations and their contribution to the heritable risk of colorectal cancer. Nature communications 7, 11883, https://doi.org/10.1038/ncomms11883 (2016).
Betel, D., Koppal, A., Agius, P., Sander, C. & Leslie, C. Comprehensive modeling of microRNA targets predicts functional non-conserved and non-canonical sites. Genome Biol 11, R90, https://doi.org/10.1186/gb-2010-11-8-r90 (2010).
Agarwal, V., Bell, G. W., Nam, J. W. & Bartel, D. P. Predicting effective microRNA target sites in mammalian mRNAs. Elife 4, https://doi.org/10.7554/eLife.05005 (2015).
Dayem Ullah, A. Z., Lemoine, N. R. & Chelala, C. A practical guide for the functional annotation of genetic variations using SNPnexus. Briefings in bioinformatics 14, 437–447, https://doi.org/10.1093/bib/bbt004 (2013).
Andersson, R. et al. An atlas of active enhancers across human cell types and tissues. Nature 507, 455–461, https://doi.org/10.1038/nature12787 (2014).
Lizio, M. et al. Gateways to the FANTOM5 promoter level mammalian expression atlas. Genome Biol 16, 22, https://doi.org/10.1186/s13059-014-0560-6 (2015).
Consortium, F. et al. A promoter-level mammalian expression atlas. Nature 507, 462–470, https://doi.org/10.1038/nature13182 (2014).
Rimmer, A. et al. Integrating mapping-, assembly- and haplotype-based approaches for calling variants in clinical sequencing applications. Nature genetics 46, 912–918, https://doi.org/10.1038/ng.3036 (2014).
Wang, K., Li, M. & Hakonarson, H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic acids research 38, e164, https://doi.org/10.1093/nar/gkq603 (2010).
Lek, M. et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 536, 285–291, https://doi.org/10.1038/nature19057 (2016).
Fu, W. et al. Analysis of 6,515 exomes reveals the recent origin of most human protein-coding variants. Nature 493, 216–220, https://doi.org/10.1038/nature11690 (2013).
Genomes Project, C. et al. A global reference for human genetic variation. Nature 526, 68–74, https://doi.org/10.1038/nature15393 (2015).
Liu, X., Wu, C., Li, C. & Boerwinkle, E. dbNSFPv3.0: A One-Stop Database of Functional Predictions and Annotations for Human Nonsynonymous and Splice-Site SNVs. Human mutation 37, 235–241, https://doi.org/10.1002/humu.22932 (2016).
Smigielski, E. M., Sirotkin, K., Ward, M. & Sherry, S. T. dbSNP: a database of single nucleotide polymorphisms. Nucleic acids research 28, 352–355 (2000).
Kircher, M., Witten, D. M., Jain, P., O’Roak, B. J. & Cooper, G. M. A general framework for estimating the relative pathogenicity of human genetic variants. 46, 310–315 https://doi.org/10.1038/ng.2892 (2014).
Ward, L. D. & Kellis, M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic acids research 40, D930–934, https://doi.org/10.1093/nar/gkr917 (2012).
Boyle, A. P. et al. Annotation of functional variation in personal genomes using RegulomeDB. Genome Res 22, 1790–1797, https://doi.org/10.1101/gr.137323.112 (2012).
Fu, Y. et al. FunSeq. 2: a framework for prioritizing noncoding regulatory variants in cancer. Genome Biol 15, 480, https://doi.org/10.1186/s13059-014-0480-5 (2014).
Dimitrieva, S. & Bucher, P. UCNEbase–a database of ultraconserved non-coding elements and genomic regulatory blocks. Nucleic acids research 41, D101–109, https://doi.org/10.1093/nar/gks1092 (2013).
Wei, Y. et al. SEA: a super-enhancer archive. Nucleic acids research 44, D172–179, https://doi.org/10.1093/nar/gkv1243 (2016).
Khan, A. & Zhang, X. dbSUPER: a database of super-enhancers in mouse and human genome. Nucleic acids research 44, D164–171, https://doi.org/10.1093/nar/gkv1002 (2016).
Pruim, R. J. et al. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics 26, 2336–2337, https://doi.org/10.1093/bioinformatics/btq419 (2010).
Arnold, M., Raffler, J., Pfeufer, A., Suhre, K. & Kastenmuller, G. SNiPA: an interactive, genetic variant-centered annotation browser. Bioinformatics 31, 1334–1336, https://doi.org/10.1093/bioinformatics/btu779 (2015).
Rosenbloom, K. R. et al. The UCSC Genome Browser database: 2015 update. Nucleic acids research 43, D670–681, https://doi.org/10.1093/nar/gku1177 (2015).
Severin, J. et al. Interactive visualization and analysis of large-scale sequencing datasets using ZENBU. Nature biotechnology 32, 217–219, https://doi.org/10.1038/nbt.2840 (2014).
Wu, C., Jin, X., Tsueng, G., Afrasiabi, C. & Su, A. I. BioGPS: building your own mash-up of gene annotations and expression profiles. Nucleic acids research 44, D313–316, https://doi.org/10.1093/nar/gkv1104 (2016).
Uhlen, M. et al. Proteomics. Tissue-based map of the human proteome. Science 347, 1260419, https://doi.org/10.1126/science.1260419 (2015).
Landrum, M. J. et al. ClinVar: public archive of relationships among sequence variation and human phenotype. Nucleic acids research 42, D980–985, https://doi.org/10.1093/nar/gkt1113 (2014).
Chubb, D., Broderick, P., Dobbins, S. E. & Houlston, R. S. CanVar: A resource for sharing germline variation in cancer patients. F1000Research 5, 2813, https://doi.org/10.12688/f1000research.10058.1 (2016).
Gao, J. et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Science signaling 6, pl1, https://doi.org/10.1126/scisignal.2004088 (2013).
Forbes, S. A. et al. The Catalogue of Somatic Mutations in Cancer (COSMIC). Current protocols in human genetics Chapter 10(Unit10), 11, https://doi.org/10.1002/0471142905.hg1011s57 (2008).
Zhang, J. et al. International Cancer Genome Consortium Data Portal–a one-stop shop for cancer genomics data. Database: the journal of biological databases and curation 2011, bar026, https://doi.org/10.1093/database/bar026 (2011).
Gonzalez-Perez, A. et al. IntOGen-mutations identifies cancer drivers across tumor types. Nature methods 10, 1081–1082, https://doi.org/10.1038/nmeth.2642 (2013).
Thorvaldsdottir, H., Robinson, J. T. & Mesirov, J. P. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Briefings in bioinformatics 14, 178–192, https://doi.org/10.1093/bib/bbs017 (2013).
Kim, Y. H., O’Neill, H. M. & Whitehead, J. P. Carboxypeptidase X-1 (CPX-1) is a secreted collagen-binding glycoprotein. Biochem Biophys Res Commun 468, 894–899, https://doi.org/10.1016/j.bbrc.2015.11.053 (2015).
Kim, Y. H. et al. Identification of carboxypeptidase X (CPX)-1 as a positive regulator of adipogenesis. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 30, 2528–2540, https://doi.org/10.1096/fj.201500107R (2016).
Wood, W. M. et al. PPARgamma Promotes Growth and Invasion of ThyroidCancer Cells. PPAR research 2011, 171765, https://doi.org/10.1155/2011/171765 (2011).
Uehiro, N. et al. Circulating cell-free DNA-based epigenetic assay can detect early breast cancer. Breast cancer research: BCR 18, 129, https://doi.org/10.1186/s13058-016-0788-z (2016).
Lindqvist, B. M., Wingren, S., Motlagh, P. B. & Nilsson, T. K. Whole genome DNA methylation signature of HER2-positive breast cancer. Epigenetics 9, 1149–1162, https://doi.org/10.4161/epi.29632 (2014).
Goedert, L. et al. Identification of Long Noncoding RNAs Deregulated in Papillary Thyroid Cancer and Correlated with BRAFV600E Mutation by Bioinformatics Integrative Analysis. Sci Rep 7, 1662, https://doi.org/10.1038/s41598-017-01957-0 (2017).
Weitzel, J. N., Blazer, K. R., MacDonald, D. J., Culver, J. O. & Offit, K. Genetics, genomics, and cancer risk assessment: State of the Art and Future Directions in the Era ofPersonalized Medicine. CA: a cancer journal for clinicians 61, 327–359, https://doi.org/10.3322/caac.20128 (2011).
Horn, S. et al. TERT promoter mutations in familial and sporadic melanoma. Science 339, 959–961, https://doi.org/10.1126/science.1230062 (2013).
Shi, J. et al. Rare missense variants in POT1 predispose to familial cutaneous malignant melanoma. Nature genetics 46, 482–486, https://doi.org/10.1038/ng.2941 (2014).
Palles, C. et al. Germline mutations affecting the proofreading domains of POLE and POLD1 predispose to colorectal adenomas and carcinomas. Nature genetics 45, 136–144, https://doi.org/10.1038/ng.2503 (2013).
Segui, N. et al. Germline Mutations in FAN1 Cause Hereditary Colorectal Cancer by Impairing DNA Repair. Gastroenterology 149, 563–566, https://doi.org/10.1053/j.gastro.2015.05.056 (2015).
Rotunno, M. et al. Whole exome sequencing in families at high risk for Hodgkin lymphoma: identification of a predisposing mutation in the KDR gene. Haematologica 101, 853–860, https://doi.org/10.3324/haematol.2015.135475 (2016).
Gara, S. K. et al. Germline HABP2 Mutation Causing Familial Nonmedullary Thyroid Cancer. The New England journal of medicine 373, 448–455, https://doi.org/10.1056/NEJMoa1502449 (2015).
White, A. E. & Harper, J. W. Cancer. Emerging anatomy of the BAP1 tumor suppressor system. Science 337, 1463–1464, https://doi.org/10.1126/science.1228463 (2012).
Jensen, M. R. & Helin, K. OVCA1: emerging as a bona fide tumor suppressor. Genes & development 18, 245–248, https://doi.org/10.1101/gad.1184004 (2004).
Reineke, E. L. & Kao, H. Y. PML: An emerging tumor suppressor and a target with therapeutic potential. Cancer therapy 7, 219–226 (2009).
Vaz, C. V. et al. The Emerging Role of Regucalcin as a Tumor Suppressor: Facts and Views. Current molecular medicine 16, 607–619 (2016).
Zhao, X., Li, X. & Zhang, X. HABP2 Mutation and Nonmedullary Thyroid Cancer. The New England journal of medicine 373, 2084, https://doi.org/10.1056/NEJMc1511631#SA1 (2015).
Offit, K. The future of clinical cancer genomics. Seminars in oncology 43, 615–622, https://doi.org/10.1053/j.seminoncol.2016.10.002 (2016).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760, https://doi.org/10.1093/bioinformatics/btp324 (2009).
Petrovski, S., Wang, Q., Heinzen, E. L., Allen, A. S. & Goldstein, D. B. Genic intolerance to functional variation and the interpretation of personal genomes. PLoS Genet 9, e1003709, https://doi.org/10.1371/journal.pgen.1003709 (2013).
Cooper, G. M. et al. Distribution and intensity of constraint in mammalian genomic sequence. Genome Res 15, 901–913, https://doi.org/10.1101/gr.3577405 (2005).
Siepel, A. et al. Evolutionarily conserved elements in vertebrate, insect, worm, and yeast genomes. Genome Res 15, 1034–1050, https://doi.org/10.1101/gr.3715005 (2005).
Pollard, K. S., Hubisz, M. J., Rosenbloom, K. R. & Siepel, A. Detection of nonneutral substitution rates on mammalian phylogenies. Genome Res 20, 110–121, https://doi.org/10.1101/gr.097857.109 (2010).
Kumar, P., Henikoff, S. & Ng, P. C. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nature protocols 4, 1073–1081, https://doi.org/10.1038/nprot.2009.86 (2009).
Adzhubei, I., Jordan, D. M. & Sunyaev, S. R. Predicting functional effect of human missense mutations using PolyPhen-2. Current protocols in human genetics Chapter 7 (Unit7), 20, https://doi.org/10.1002/0471142905.hg0720s76 (2013).
Quinlan, A. R. & Hall, I. M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842, https://doi.org/10.1093/bioinformatics/btq033 (2010).
Quinlan, A. R. BEDTools: The Swiss-Army Tool for Genome Feature Analysis. Current protocols in bioinformatics/editoral board, Andreas D. Baxevanis… [et al.]47, 11 12 11-34 https://doi.org/10.1002/0471250953.bi1112s47 (2014).
Consortium, E. P. et al. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature 447, 799–816, https://doi.org/10.1038/nature05874 (2007).
Chun, S. & Fay, J. C. Identification of deleterious mutations within three human genomes. Genome Res 19, 1553–1561, https://doi.org/10.1101/gr.092619.109 (2009).
Schwarz, J. M., Rodelsperger, C., Schuelke, M. & Seelow, D. MutationTaster evaluates disease-causing potential of sequence alterations. Nature methods 7, 575–576, https://doi.org/10.1038/nmeth0810-575 (2010).
Reva, B., Antipin, Y. & Sander, C. Predicting the functional impact of protein mutations: application to cancer genomics. Nucleic acids research 39, e118, https://doi.org/10.1093/nar/gkr407 (2011).
Shihab, H. A. et al. Predicting the functional, molecular, and phenotypic consequences of amino acid substitutions using hidden Markov models. Human mutation 34, 57–65, https://doi.org/10.1002/humu.22225 (2013).
Carter, H., Douville, C., Stenson, P. D., Cooper, D. N. & Karchin, R. Identifying Mendelian disease genes with the variant effect scoring tool. BMC Genomics 14(Suppl 3), S3, https://doi.org/10.1186/1471-2164-14-S3-S3 (2013).
Choi, Y., Sims, G. E., Murphy, S., Miller, J. R. & Chan, A. P. Predicting the functional effect of amino acid substitutions and indels. PLoS One 7, e46688, https://doi.org/10.1371/journal.pone.0046688 (2012).
Calabrese, R., Capriotti, E., Fariselli, P., Martelli, P. L. & Casadio, R. Functional annotations improve the predictive score of human disease-related mutations in proteins. Human mutation 30, 1237–1244, https://doi.org/10.1002/humu.21047 (2009).
Acknowledgements
We thank the DKFZ Genomics and Proteomics Core Facility for Illumina Sequencing Services and Data Management Group (DMG) in the Division of Theoretical Bioinformatics, DKFZ for managing the NGS data. We thank Transcan ERA-NET funding from the German Federal Ministry of Education and Research (BMBF).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: A.K., O.R.B., K.H. and A.F. Performed the experiments: A.K. O.R.B., N.P., A.F. Analyzed the data: A.K. O.R.B., N.P. S.G., C.D., E.B., R.E., M.S., K.H. and A.F. Contributed to the writing of the manuscript: A.K., K.H. and A.F. All authors have approved final version of the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kumar, A., Bandapalli, O.R., Paramasivam, N. et al. Familial Cancer Variant Prioritization Pipeline version 2 (FCVPPv2) applied to a papillary thyroid cancer family. Sci Rep 8, 11635 (2018). https://doi.org/10.1038/s41598-018-29952-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-29952-z
This article is cited by
-
The complementary roles of genome-wide approaches in identifying genes linked to an inherited risk of colorectal cancer
Hereditary Cancer in Clinical Practice (2023)
-
Cpxm2 as a novel candidate for cardiac hypertrophy and failure in hypertension
Hypertension Research (2022)
-
Whole exome sequencing identifies novel germline variants of SLC15A4 gene as potentially cancer predisposing in familial colorectal cancer
Molecular Genetics and Genomics (2022)
-
Genome-wide interaction study with major depression identifies novel variants associated with cognitive function
Molecular Psychiatry (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.