Abstract
From the wild-type strain Steptomyces sp. AK 671, three nitrogen-containing octaketides were isolated, bhimamycins F, H and I, besides the known azaanthraquinone utahmycin A and polyketide shunt products SEK 4, SEK 4b, mutactin, dehydromutactin and EM18. The structures were characterized by MS and NMR experiments. The hitherto unknown absolute configuration of the two enantiomers of EM18 was determined by online-CD spectroscopy and quantum-chemical CD calculations. Bhimamycins H and I show weak antibacterial activities, whereas the enzyme phosphodiesterase 4 is strongly inhibited by bhimamycins H and I, which has never been reported for nitrogen-containing octaketides. In addition, bhimamycin H inhibits the enzyme glycogen synthase kinase-3β.
Similar content being viewed by others
Introduction
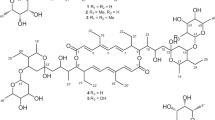
The alkaliphilic strain Streptomyces sp. AK 671 is a potent producer of various intermediates and end-products in the biosynthetic pathway to aromatic polyketides.2 It has been shown that the octaketide chrysophanol glucuronide (4) originates from one acetyl-CoA and seven malonyl-CoA units by an unusual folding mode S′ of the polyketide chain and cyclization to the bicyclic diketo precursor genoketide A1 (the Harris–Franck diketone, 1),3 then glucuronidation to genoketide A2 (2) and ring closure to prechrysophanol glucuronide (3),4 as shown in Figure 1.
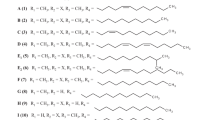
Astonishingly, strain Streptomyces sp. AK 671 accumulated the intermediates 1–3 in high concentrations, which permitted their isolation in substantial quantities.2 Besides 1–4, several other aromatic polyketides were produced by the strain depending on the cultivation conditions. In a previous publication,2 we reported on the isolation and characterization of the polyketide synthase (PKS) shunt products BSM1 (5) and 3,8-dihydroxy-1-methylanthraquinone-carboxylic acid (DMAC, 6), which were originally isolated from genetically engineered Streptomyces strains blocked in the biosynthesis of actinorhodin.5, 6 Continued studies with strain AK 671 resulted in the detection and characterization of a series of further PKS shunt products such as SEK 4 (7), SEK 4b (8), mutactin (9), dehydromutactin (10) and EM18 (11) as a mixture of all four possible stereoisomers. The structures of 5–11 are summarized in Figure 2.
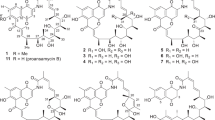
In our previous studies, we isolated and characterized the main polyketides 1–4 and, furthermore, the octaketides bhimamycins A (12) and B (13) from the culture filtrate of strain AK 671. This extraordinary, biosynthetically talented wild-type Streptomyces strain additionally produced the known nitrogen-containing octaketidic compound utahmycin A (14),7 the hitherto only synthetically prepared bhimamycin F (15),8 and the new bhimamycins H (16) and I (17) with UV-visible absorption characteristics of anthraquinones. Their fermentation, isolation, structure determination and biological activities are reported in this publication. The structures of octaketides 12–17 are shown in Figure 3.
Results
Fermentation and isolation
Bhimamycins F (15), H (16) and I (17) were isolated as minor congeners produced by strain AK 671 at a fermentation time of 72 h when the strain was grown in a 10-l fermentor in complex medium SGG. They were gained from the culture filtrate by ethyl acetate extraction and purified by column chromatography using diol-modified silica gel, Sephadex LH-20 and Toyopearl HW-40 F. Compounds 15, 16 and 17 were obtained in amounts of 2 mg, 9 mg and 18 mg, respectively, all as yellowish powders after lyophilization.
Structure elucidation
The structures of the known compounds, 5–14, were determined by mass and NMR spectra, and by comparison with the spectroscopic and physical data from the literature. Interestingly, the shunt product EM18 (11, Figure 2) was detected 'twice', in a ratio of ca. 6:1 (41 mg, 11a, and 6.5 mg, 11b), in the culture filtrate, eluting baseline separated during HPLC on an achiral reverse-phase adsorbant. The nearly identical 1D- and 2D-NMR data (for the complete NMR data see Supplementary Tables S1 and S2) delivered the same constitution for both substances. EM18, bearing two stereogenic centers at C-6 and C-15, has been known for a long time,9 and its biosynthetic origin has been intensely studied,5, 10, 11 but never has the relative or the absolute configuration been discussed. Obviously Streptomyces sp. AK 671 produced both diastereomeric forms of EM18, the unlike-diastereomer12 (that is, R/S or S/R) and the like-diastereomer (that is, R/R or S/S). As standard NOE experiments (1 sec mixing time) only delivered quite identical results for both diastereomers, extended NOE experiments were performed to provide information on the relative configuration. Transient NOE enhancements permit estimation of internuclear distances by variation of the mixing time.13 In our case, multiple NOE spectra with increasing mixing times (50, 100, 200 and 400 ms) were taken. Compound 11a had an NOE correlation between H-6 and Ha-14 at all mixing times, whereas compound 11b showed this correlation only in the case of the longer mixing times (200 and 400 ms). This correlation can only be seen when the respective protons (in yellow) are both axial, which means that the pyranone substituent must occupy an equatorial orientation (Figure 4).
NOESY correlations between Ha-14 and H-6 in the diastereomers of EM18, 11a (rapid, unlike, above), and 11b (slow, like, below). Arbitrarily the 6S, 15R-enantiomer for unlike and the 6S, 15S-enantiomer for like are shown. Conformational analyses were done by SCS-MP2/def2-TZVP//B97D/SVP and COSMO calculations.
It seems that in case of compound 11b, this equatorial position is energetically less favored and, hence, less populated in solution than the axial orientation. As a consequence, the NOE correlation can only be seen in longer mixing times. By contrast, this is not the case for 11a, where the correlation can be found at all mixing times. A conformational analysis using quantum-chemical calculations (SCS-MP2/def2-TZVP//B97D/SVP and COSMO[acetone]) of all possible diastereomers of 11 showed that significant conformational differences are to be expected between the like and the unlike configurations. In the like configuration, the axial position of the pyranone is highly favored and the equatorial position is nearly not populated (94% axial, only 6% equatorial), in contrast to the unlike configuration, where the equatorial orientation is significantly more populated (79% axial, 21% equatorial). Consequently, compound 11a is either R/S- or S/R-configured (unlike) and 11b R/R or S/S.
To establish the absolute configuration, CD spectra were taken of the two diastereomers, but neither 11a nor 11b delivered any CD effect, suggesting that both compounds were racemic mixtures. A successful resolution by HPLC on a chiral column revealed that each diastereomer consisted of two enantiomers in an approximately 1:1 ratio and permitted measurement of the CD spectra of all of the enantiomers online, in the stopped-flow mode. By application of the Exciton Chirality method,14 it was now possible to assign the absolute configuration of all four stereoisomers of 11. The CD spectra of the enantiomers of 11a and 11b show all an exciton couplet at 270 nm. This couplet arises from an interaction of the pyranone chromophore with the phenolic one. In the case of a 6S-configuration, a positive couplet results from the orientation of the respective transition dipole moments (Figure 5), whereas a 6R-configuration gives a negative couplet. Thus, peak A of 11a with its negative couplet is 6R,15S and peak B, with the positive Cotton effect is 6S,15R-configured and for 11b peak A is 6S,15S-configured (Figure 5), whereas peak B has the 6R,15R configuration.
For compound 15, a molecular formula of C22H17NO6 was cald from its exact mass of 390.09821 ([M-H]−). In the 1H NMR, the signals for seven aromatic protons, one proton in the shift range of an R2-CH-O fragment and two methyl groups were visible. The aromatic protons were assigned by 1H-1H-COSY and NOESY correlations (Figure 6b) to correspond to an ABC spin system and to a further system of four consecutive aromatic protons. The 13C NMR data gave hints at 16 sp2 carbons, two keto functions, one carboxy function, one saturated carbon attached to a heteroatom and two methyl groups (Table 1; for the complete NMR data, see Supplementary Table S3). In a series of HMBC experiments, a structure consisting of a utahmycin A-related part and a benzoic acid derivative part was deduced (Figure 6a). In comparison with utahmycin A (14, Figure 3),7 the NMR signals of one aromatic carbon and one proton in the three-ring skeleton of compound 15 were missing, suggesting that one of the aromatic rings was only five-membered. As the three protons and the hydroxy function of the western part were preserved, the aromatic system containing the nitrogen had to be different. By comparison with data from the literature, compound 15 was identified to belong to the class of the bhimamycins (Figure 3).8
As compound 15 carried a hydroxy group at C-10 (Figure 6a), it seemed to be a follow-up product of bhimamycin A (12). The attached benzoic acid derivative was found to be anthranilic acid, linked to the 2H-benzo[f]isoindole-4,9-dione at the ortho position. Due to the increased steric hindrance of the carboxylic acid moiety the N,C-axis is rotationally somewhat hindered, yet slowly rotating, creating two sets of co-occurring proton and carbon signals for the two configurationally semi-stable atropo-diastereomers 15 in the NMR spectrum (Table 1). Compound 15, previously obtained as a side product in the chemical synthesis of bhimamycin D, has now been isolated for the first time as a natural product from a living organism. It was named bhimamycin F.8
Compound 16 exhibited an exact mass of 390.09596 ([M+H]+), corresponding to an elemental composition of C22H15NO6. The proton NMR experiments evidenced the presence of three neighboring aromatic protons and two methyl groups, and an additional A2B2 spin system. In the 13C NMR spectrum, the signals of 14 aromatic carbons, two methyl groups, three keto functions and one carboxy function were observed. The intensities of two of the aromatic carbon signals were twice as high as those of the others, possibly representing two chemically identical carbons, each (Table 2; for the complete NMR data see Supplementary Table S4). From the similarity of its NMR data with those of bhimamycin F (15), compound 16 might be related to bhimamycin B because of its keto function at C-10. As the mass was similar to that of 15, the attached aromatic unit had to be anthranilic acid or a close analog, like para-aminobenzoic acid. The reduced set of signals in the 1H and 13C NMR spectra indicated a symmetric structure in the phenyl ring, thus excluding anthranilic acid. The complete structure was assigned by NOE and HMBC experiments (Figure 7a and b), evidencing 16 to be a product of bhimamycin B (13) and para-aminobenzoic acid. It was thus a new compound, now named bhimamycin H.
For compound 17, a molecular formula of C17H15NO4 was deduced by high-resolution ESI MS ([M+Na]+ m/z 320.08911). In the 1H NMR spectrum signals for three neighboring aromatic protons, one alkoxy or acetalic proton, four alkyl protons, forming high-order multiplets, and two methyl groups were observed. The 13C NMR and the DEPT spectra showed the presence of two keto groups, seven quaternary sp2-carbon atoms, six aromatic CH functions, two methylene units, one alkoxy carbon and two methyl groups (Table 3; for the complete NMR data, see Supplementary Table S5). Again, a bhimamycin-like structure was assumed, in this case resembling that of the known bhimamycin C (18, Figure 8),8 previously isolated as a natural product from a Streptomyces strain.
In contrast to bhimamycin C the 2′-methylene unit of 17 carried two diastereotopic protons. As 18 had a mass of 315 kDa, which is the MW of 17 (298 kDa) plus that of water (H2O=18 kDa), the new compound was proposed to be the condensed cyclic form of bhimamycin C. This assumption was unambiguously confirmed by HMBC and NOESY experiments (Figure 9a and b). Of significance were the HMBC correlations from Ha-2′ and Hb-2′ to C-10, and the NOESY interactions of Ha-2′ and Hb-2′ with H-10 and Me-11. Bhimamycin I (17) did not deliver a measurable αD or CD effect (for the physicochemical properties of compounds 15–17, see Table 4), leaving the assumption that it might be a racemic mixture. However, resolution of the enantiomers on a chiral phase did not succeed.
Biological activities
Compounds 14, 16 and 17 showed weak activities against S. epidermidis with IC50 values of 11 μM, 14.6 μM and 88.9 μM, respectively. Interestingly, only compound 16 affected the growth of methicillin-resistant Staphylococcus aureus, with an IC50 value of 13.5 μM. The activity of the enzyme GSK-3β15, 16 was inhibited by compound 16 (IC50 value of 18 μM). The IC50 values of the inhibitory activity against phosphodiesterase 4 of compounds 14, 16 and 17 were >30 μM, 7.65 μM and 6.05 μM, respectively.
Discussion
The pattern of polyketide metabolites from Streptomyces sp. AK 671 changed significantly in dependence on the fermentation conditions, in particular on the medium constituents and the type of cultivation vessel. The conversion of the octaketidic intermediate genoketide A1 (1) via genoketide A2 (2) and prechrysophanol glucuronide (3) to the end product chrysophanol glucuronide (4) was observed preferably in the complex medium SGG. Interestingly, the strain accumulated the three intermediates in high amounts and then converted them one by one, eventually producing 4.2 Besides compounds 1–4 as the main polyketide products, strain AK 671 additionally produced seven members of so-called 'metabolic shunt products', 5–11, as minor compounds.
Polyketide shunt products are usually found as spontaneously cyclized intermediates that cannot be enzymatically transformed to their biosynthetic end-product (for example, to actinorhodin), as a consequence of the inactivation of genes in the biosynthetic pathway.17 The truncated hexaketide BSM1 (5) was described as a shunt product and not catabolic metabolite from the actinorhodin-deficient recombinant host S. lividans K4-114.5 The octaketides SEK 4 (7) and SEK 4b (8) were discovered in actIII ketoreductase mutants of S. coelicolor A3(2),18 whereas mutactin (9), dehydromutactin (10) and EM18 (11) were found to be accumulated in actVII aromatase deficient mutants of S. coelicolor A3(2).5, 9 The fully aromatized octaketide DMAC (6) was isolated from actIV mutants, and a spontaneous cyclization of the third carbocyclic ring was postulated to build up the anthraquinone system of DMAC followed by a decarboxylation step to yield aloesaponarin II.19 Even though DMAC was detected in strain AK 671,2 no aloesaponarin II was detected under any cultivation condition. It can be speculated that the huge number of polyketide shunt products in cultures of the wild-type Streptomyces strain AK 671 is caused by a high production level and accumulation of non- or partially-aromatized intermediates leading to genoketide A1 (1) up to a concentration of 390 mg l−1.2 This precursor is then, at a slow rate, enzymatically transformed via 2 and 3 to the octaketidic end-product chrysophanol glucuronide (4).
In contrast to the well-investigated hexaketidic naphthylisoquinoline alkaloids from tropical plants,20, 21 only a few members of nitrogen-containing octaketides have so far been described as natural products. While several 3-methyl-2-azaanthraquinone derivatives have been isolated from lichens and fungi, utahmycin A (14) is the only natural precedent of the 1,3-dimethyl-substituted 2-azaanthraquinones, previously isolated from cultures of Streptomyces albus transformed with an environmental DNA clone by Bauer et al.7 They postulated that utahmycin A may arise from erdacin polyketide intermediates originating through a folding mode S of the octaketidic chain (actually mode S', as defined earlier4), that is, in a Streptomyces-specific way.7, 22 In Streptomyces AK 671, utahmycin A (14) might be formed from the known chrysophanol precursor genoketide A1 (1; Figure 1) just by nitrogen incorporation and oxidation to the azaanthraquinone. The diketone 1 (like also compounds 2–4) are formed through the S′-type folding, so that compound 14 should originate from the S′-mode, too, as also postulated by Bauer et al.7 However, the question whether utahmycin A is a shunt product in the polyketide biosynthesis or not—or even an artefact, given the easy spontaneous incorporation of ammonia into monocyclic diketones related to 123—remains open.
Bhimamycins F (15), H (16) and I (17) are characterized by a benz[g]isoindolo-4,9-dione chromophore, which was first described in bhimamycins C and D. These metabolites have so far been isolated from Streptomyces sp. GW32/698 besides bhimamycins A and B.8 As reported earlier, bhimamycins A and B were also found and isolated from Streptomyces strain AK 671.2 Bhimamycin F (15) can be considered as a product of bhimamycin A and anthranilic acid, and bhimamycin H (16) as the product of bhimamycin B and para-aminobenzoic acid. Furthermore, compound 17 could represent the condensed cyclic form of bhimamycin C. Whether or not compounds 15–17 are true natural products cannot be proven. Due to the low quantities, they are neither detectable in the culture filtrate nor in the mycelium extracts, in contrast to bhimamycins A and B. The presence of anthranilic acid and para-aminobenzoic acid as constituents of the production medium was excluded by HPLC-DAD analysis, and thus, a chemical reaction with bhimamycins A or B is considered to be unlikely. A further argument for the natural origin of the two compounds is the fact that the other products—bhimamycin A and para-amino benzoic acid, and bhimamycin B and anthranilic acid—were not found. If the formation of 15 and 16 were the result of random chemistry in the fermentation broth, the mentioned combination products should occur in comparable amounts, too.
The origin of compound 17 as a genuine natural product, by contrast, is indeed questionable. If bhimamycin C (18, Figure 8) is produced by the Streptomyces strain, the conditions during workup—aqueous, acidic—will favor the spontaneous formation of a stabilized heterobenzylic cation, and, subsequently, the cyclization to bhimamycin I, which possesses a stable six-membered ring structure (see Supplementary Information: considerations on the native origin of bhimamycin I).
Utahmycin A (14) and bhimamycin H (16) showed weak, bhimamycin I (17) very weak activities against S. epidermidis, a human-pathogenic bacterium. The methicillin-resistant S. aureus strain was inhibited only by bhimamycin H (16). In an earlier study, antibacterial activity against S. aureus had been observed for bhimamycins A (12), B (13) and E at a concentration of 20 μg per disc,8 whereas genoketides A1 (1) and A2 (2) had shown no antibacterial activities up to concentrations of 1 mg ml−1.2 Utahmycin A (14), bhimamycin H (16) and bhimamycin I (17) were active against the enzyme phosphodiesterase 4 (PDE4), bhimamycin H (16) inhibited the activity of the glycogen synthase kinase-3β (GSK-3β). To our knowledge, this is the first report on nitrogen-containing octaketides showing an inhibition of PDE4. Investigations on further, similar compounds, like derivatives of bhimamycins H and I, could be of interest for the discovery of new drugs for the treatment of the chronic obstructive pulmonary disease.24
The diversity and multitude of aromatic polyketides and polyketide shunt products isolated from the alkaliphilic Streptomyces AK 671 are unprecedented for a wild-type Streptomyces strain. The fermentation and growth conditions strongly influenced the accumulation of metabolites, resulting in the isolation and structure elucidation of new polyketides related to chrysophanol and bhimamycins. The described chrysophanol-related compounds gave unique insight into the biosynthesis of aromatic octaketides including final products from main metabolic pathways and side routes. The secondary metabolites from the bhimamycin class showed the first reported inhibition of PDE4 by a nitrogen-containing octaketide. The results encourage the search for further novel bioactive natural compounds produced by bacteria of the order Actinomycetales, in particular of the genus Streptomyces.
Experimental Section
HPLC-diode array analysis
The HPLC-DAD system consisted of an HP 1090M LC equipped with a diode array detector, an HP Kayak XM 600 ChemStation and HPLC software revision A.08.03 (Agilent Technologies, Waldbronn, Germany). Multiple-wavelength monitoring was performed at 210, 230, 260, 280, 310, 435 and 500 nm, and UV-visible spectra were measured from 200 to 600 nm. Sample preparation and chromatographic conditions were performed as described earlier.25 Evaluation of the chromatograms was done by means of an in-house HPLC-UV-Vis database containing about 960 entries, mostly antibiotics.26
Fermentation and isolation
For production of compounds 5–14, strain AK 671 was cultivated in a 10-l stirred tank fermentor (Biostat S, B. Braun, Melsungen, Germany) in the complex SGG medium that consisted of starch soluble 10 g, glucose 10 g, glycerol 10 g, Bacto peptone 5 g (Becton Dickinson, Franklin Lakes, NJ, USA), corn steep powder 2.5 g (Marcor, Carlstadt, NJ, USA), yeast extract 2 g (Ohly Kat, Deutsche Hefewerke, Hamburg, Germany), NaCl 1 g in 1 l tap water. The pH was adjusted to 7.3 (5 M NaOH) prior to sterilization. The fermentation was carried out at 27 °C for 96 h with an aeration rate of 0.5 volume air per volume per min, and an agitation of 250 r.p.m. The isolation of compounds 5–14 from the culture filtrate was done in a succession of ethyl acetate extraction and size-exclusion column chromatography on Sephadex LH-20 (Amersham, Freiburg, Germany) and Toyopearl HW-40-F (Toyo Biosep, Stuttgart, Germany) in MeOH. For production of compounds 15–17, batch fermentations of strain AK 671 were carried out in a 10-l stirred tank fermenter in SGG medium. The fermentor was inoculated with 5 vol-% of a shake pre-culture grown in the same medium in 500-ml Erlenmeyer flasks with one baffle for 40 h on a rotary shaker at 120 r.p.m. at 27 °C. The fermentation was carried out for 72 h at 27 °C with an agitation of 300 r.p.m. and an aeration of 0.5 volume air per volume per min. Hyflo Super-cel (2%) was added to the fermentation broth, which was separated by multiple sheet filtration; the mycelium was discarded. The culture filtrate (8.5 l) was adjusted to pH 5.0 (1 M HCl) and extracted three times with EtOAc. The organic extracts were combined and concentrated to dryness in vacuo. The crude extract was subjected to a diol-modified silica gel column (40 cm × 3.6 cm; LiChroprep Diol; E. Merck, Darmstadt, Germany) and separation was accomplished by a linear gradient from CH2Cl2 to CH2Cl2-MeOH (9:1) within 4 h at a flow rate of 9 ml min−1. Fractions containing 15, 16 and 17 were combined and purified by chromatography on Sephadex LH-20 (90 cm × 2.5 cm) and Toyopearl HW-40-F (90 cm × 2.5 cm) using MeOH as the eluent.
Structure elucidation
NMR spectra were recorded on a Bruker DMX 600 spectrometer (Bruker, Rheinstetten, Germany) at ambient temperature. The chemical shifts are given in δ units (p.p.m.) taking the signals of the deuterated solvents as internal reference for 1H and 13C NMR spectroscopy; the coupling constants J are given in Hertz (Hz). For basic NOESY experiments the mixing time was set to 1 s, for extended NOESY experiments the mixing time was set to 50, 100, 200 and 400 ms. Proton-detected, heteronuclear correlations were analyzed using HSQC (optimized for 1JHC=145 Hz) and HMBC (optimized for nJHC=7 Hz). Mass spectral analysis was achieved on a time-of-flight mass detector micrOTOF II focus (Bruker Daltonics, Bremen, Germany).
HPLC on a chiral stationary phase was carried out on a Lux Cellulose-1 column (0.5 μm; 250 mm × 4.6 mm; Phenomenex, Torrance, CA, USA) with a flow rate of 0.8 ml min−1 and an isocratic solvent system: solvent (A) H2O (+0.05% trifluoroacetic acid), and solvent (B) CH3CN (+0.05% trifluoroacetic acid), 18% of solvent A. Online-CD spectra were recorded in the stopped-flow mode on a JASCO J-715 circular-dichroism spectrometer (JASCO, Gross-Umstadt, Germany) equipped with a 5-mm flow cell and a BESTA motor valve (BESTA-Technik, Wilhelmsfeld, Germany).
Biological assays
The antimicrobial activity of compounds 14, 16 and 17 against S. epidermidis DSM 20044 and methicillin-resistant S. aureus DSM 18827 was measured according to Schulz et al.27 The cytotoxic activity against the cell line HepG2 and the determination of the phosphodiesterase (PDE4-4B2) inhibitory activity of compounds 14, 16 and 17 were performed according to Kim et al.28 Glycogen synthase kinase-3β (GSK-3β) inhibition was determined in an in vitro assay adapted from a luminescent assay described by Baki et al.29 To determine the IC50 values of the enzyme-inhibitory activities concentrations ranging from 0.1 μM to 50 μM were analyzed twice in duplicates.
References
Helaly, E. H. et al. Warkmycin, a novel angucycline antibiotic produced by Streptomyces sp. Acta 2930. J. Antibiot. (e-pub ahead of print 17 July 2013; doi:10.1038/ja.2013.74).
Fiedler, H.-P. et al. Genoketides A1 and A2, new octaketides and biosynthetic intermediates of chrysophanol produced by Streptomyces sp. AK 671. J. Antibiot. 61, 464–473 (2008).
Bringmann, G. et al. Different polyketide folding modes converge to an identical molecular architecture. Nature Chem. Biol. 2, 429–433 (2006).
Bringmann, G., Gulder, T. A. M., Hamm, A., Goodfellow, M. & Fiedler, H.-P. Multiple convergence in polyketide biosynthesis: a third folding mode to anthraquinone chrysophanol. Chem. Commun. 2009, 6810–6812 (2009).
Kalaitzis, J. A. & Moore, B. S. Heterologous biosynthesis of truncated hexaketides derived from the actinorhodin polyketide synthase. Nat. Prod. Rep. 67, 1419–1422 (2004).
Krupa, J., Lessmann, H. & Lackner, H. Ein α-methylanthrachinon aus streptomyceten. Liebigs Ann. Chem. 1989, 699–701 (1989).
Bauer, J. D., King, R. W. & Brady, S. F. Utahmycins A and B, azaquinones produced by an environmental DNA clone. J. Nat. Prod. 73, 976–979 (2010).
Fotso, S. et al. Bhimamycin A∼E and bhimanone: isolation, structure elucidation, and biological activity of novel quinone antibiotics from a terrestrial streptomycete. J. Antibiot. 56, 931–941 (2003).
Alvarez, M. A., Fu, H., Khosla, C., Hopwood, D. A. & Bailey, J. E. Engineered biosynthesis of novel polyketides: properties of the whiE aromatase/cyclase. Nat. Biotechnol. 14, 335–338 (1996).
Xiang, L. K., John, A. & Moore, B. S. EncM, a versatile enterocin biosynthetic enzyme involved in Favorskii oxidative rearrangement, aldol condensation, and heterocycle-forming reactions. Proc. Natl Acad. Sci. USA 101, 15609–15614 (2004).
Zhang, H.-I. et al. Mutactin, a novel polyketide from Streptomyces coelicolor. Structure and biosynthetic relationship to actinorhodin. J. Org. Chem. 55, 1682–1684 (1990).
Seebach, D. & Prelog, V. The unambiguous specification of the steric course of asymmetric syntheses. Angew. Chem. Int. Ed. Engl. 21, 654–660 (1982).
Claridge, T. D. W. High-Resolution NMR Techniques in Organic Chemistry Vol. 20, 301–306 Tetrahedron Organic Chemistry Series: Oxford, UK, (2009).
Berova, N., Polavarapu, P. L., Nakanishi, K. & Woody, R. W. Comprehensive Chiroptical Spectroscopy Vol. 2, (John Wiley & Sons: Hoboken, USA, (2012).
Cohen, P. & Frame, S. The renaissance of GSK3. Nat. Rev. Mol. Cell. Biol. 2, 769–776 (2001).
Bhat, R. V., Budd Haeberlein, S. L. & Avila, J. Glycogen synthase kinase 3: a drug target for CNS therapies. J. Neurochem. 89, 1313–1317 (2004).
Hopwood, D. A. Genetic contribution to understanding polyketide synthases. Chem. Rev. 97, 2465–2497 (1997).
Fu, H., Ebert-Koshla, S., Hopwood, D. A. & Khosla, C. Engineered biosynthesis of novel polyketides: dissection of the catalytic specificity of the act ketoreductase. J. Am. Chem. Soc. 116, 4166–4170 (1994).
Bartel, P. L. et al. Biosynthesis of anthraquinones by interspecies cloning of actinorhodin biosynthesis genes in streptomycetes: clarification of actinorhodin gene functions. J. Bacteriol. 172, 4816–4826 (1990).
Bringmann, G. & Pokorny, F. In: Cordell G. A., (Ed.) The Alkaloids Vol. 46, 127–271 Academic Press: New York, USA, (1995).
Bringmann, G., Wohlfarth, M., Rischer, H., Grüne, M. & Schlauer, J. A new biosynthetic pathway to alkaloids in plants: acetogenic isoquinolines. Angew. Chem. Int. Ed. Engl. 112, 1464–1466 (2000).
King, R. W., Bauer, J. D. & Brady, S. F. An environmental DNA-derived type II polyketide biosynthetic pathway encodes the biosynthesis of the novel pentacyclic polyketide, erdacin. Angew. Chem. Int. Ed. Engl. 48, 6257–6261 (2009).
Bringmann, G. Biomimetische Synthesen beider Molekülhälften der Ancistrocladus- und der Triphyophyllum-Alkaloide aus gemeinsamen Vorstufen. Liebigs Ann. Chem. 1985, 2126–2134 (1985).
Page, C. P. & Spina, D. Selective PDE inhibitors as novel treatments for respiratory diseases. Curr. Opin. Pharmacol. 12, 275–286 (2012).
Nachtigall, J. et al. Benzoxacystol, a benzoxazine-type enzyme inhibitor from the deep-sea strain Streptomyces sp. NTK 935. J. Antibiot. 64, 453–457 (2011).
Fiedler, H.-P. Screening for novel secondary metabolites by HPLC and UV-visible absorbance libraries. Nat. Prod. Lett. 2, 119–128 (1993).
Schulz, D. et al. Abenquines A-D: aminoquinone derivatives produced by Streptomyces sp. strain DB634. J. Antibiot. 64, 763–768 (2011).
Kim, B.-Y. et al. Elaiomycins B and C, novel alkylhydrazides produced by Streptomyces sp. BK 190. J. Antibiot. 64, 595–597 (2011).
Baki, A., Bielik, A., Molnar, L., Szendrei, G. & Keseru, G. M. A. A high throughput luminescent assay for glycogen synthase kinase-3beta inhibitors. Assay Drug Develop. Technol 5, 75–83 (2007).
Acknowledgements
We are grateful to Prof M Goodfellow, Newcastle University, for providing us the strain Streptomyces sp. AK 671. Financial support from the DFG (German Research Association, Sonderforschungsbereich 630 “Agents against Infectious Diseases”, Universiät Würzburg) and from the Ministry of Science, Economic affairs and Transport of Schleswig-Holstein, Kiel, of the project no. 12208009 “Aufbau einer Reinsubstanz-Bibliothek mariner Naturstoffe’ is gratefully acknowledged. We thank E Ruckdeschel and Dr M Grüne for the NMR experiments, F Dadrich and Dr M Büchner for the mass spectra, and F Witterauf for the measurement of the online-CD spectra (Universität Würzburg). JFI and JW are grateful to A Erhard (Kieler Wirkstoff-Zentrum) for performing the activity tests. PJ and HPF thank A Kulik (Universität Tübingen) for assistance in fermentations and HPLC-ESI-MS analysis.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Art. No. 67 in ‘Biosynthetic Capacities of Actinomycetes’. Art. No. 66: see Helaly et al.
Supplementary Information accompanies the paper on The Journal of Antibiotics website
Supplementary information
Rights and permissions
About this article
Cite this article
Jetter, P., Steinert, C., Knauer, M. et al. New bhimamycins from Streptomyces sp. AK 671. J Antibiot 66, 719–726 (2013). https://doi.org/10.1038/ja.2013.82
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2013.82