Abstract
The Clarion Clipperton Fracture Zone (CCZ) is an abyssal region in the north-east Pacific that is currently being explored for metal-rich polymetallic nodules, but also harbors a highly diverse megabenthic community. This community is influenced by multiple environmental gradients including bathymetric structures as well as differences in habitat and food availability. This study focuses on the benthic megafauna investigated in an exploration area positioned in the very east of the CCZ, which exhibits the lowest water depths (mean: 4200 m) and the highest flux of particulate organic carbon (POC) of the CCZ. Case studies using seafloor images for the detection of megafauna have revealed differences between seamounts and abyssal hills compared to nodule fields, as well as differences in the community composition between areas with and without nodule coverage and rock outcrop. Extrapolations suggest a richness of more than 300 morphotypes in the study area, including multiple invertebrate groups such as corals, sponges, echinoderms, and crustaceans as well as fish. Focusing on sampled specimens, diversities of Ophiuroidea, Porifera, and Bryozoa are high and more species are likely to be discovered in the study area. This also applies for the taxon Ophiuroidea, which is among the taxa investigated in the greatest detail so far. In the context of deep-sea mining, megafauna has been in the focus of a variety of environmental studies including baseline analyses, disturbance experiments, and/or testing of mining components or systems. These studies identify and address key factors responsible for the observed natural and impacted distribution patterns and thereby help to constrain expected anthropogenic impacts to the deep-sea environment in the context of deep-sea mining. Specifically in the area of focus of this study, 10 years of megafauna analyses have shown that the biodiversity in the selected preservation reference zone (PRZ) is not as similar to that of the impact reference zone (IRZ) as originally hypothesized based mainly on geological parameters. We suggest that recent area-wide habitat classifications and faunal mapping exercises (e.g., Uhlenkott et al. 2020, 2022) are used to designate a new PRZ that is more similar to the IRZ to meet its purpose, but that the current PRZ is maintained for scientific and conservation purposes.
Similar content being viewed by others
Introduction
The Clarion Clipperton Fracture Zone (CCZ) corresponds to a vast area of about 6 million square kilometres in the north-eastern Pacific Ocean. It is located between the Clarion and Clipperton Fracture Zones from north to south (22°N to 2.5°S) and between Mexico and Hawaii from east to west (115°W to 158°W), thereby covering approximately 1.7% of the world’s Ocean surface (Lodge et al. 2014; Glover et al. 2016). The presence of polymetallic nodules on the seafloor of this abyssal area with water depths exceeding 4000 m is of great economic interest, as they contain high grades of copper, nickel, cobalt, and rare earth elements in addition to manganese (Hein et al. 2013; Kuhn et al. 2017). However, the potentially severe impacts of future nodule mining on the environment and the fauna have strongly increased the scientific interest for this area in recent years (Wedding et al. 2013; Niner et al. 2018; Boetius and Haeckel 2018).
The seafloor of the CCZ is outside the exclusive economic zone of any country and therefore falls under the jurisdiction of the International Seabed Authority (ISA; www.isa.org.jm). The ISA has assigned 17 contracts for the exploration of polymetallic nodules in the CCZ since the year 2000. One of these contract areas with a size of ~75,000 km2 was issued to the German Federal Institute for Geoscience and Natural Resources (BGR) in 2006, comprising a western and an eastern area with a scientific focus on the eastern area, BGR-E, for which data is available from several campaigns.
Nodule fields contribute to habitat heterogeneity, which in turn considerably increases the biodiversity of benthic megafauna on the abyssal seafloor (Simon-Lledó et al. 2019a; Uhlenkott et al. under review). The local species richness can rise to above 200 morphospecies in a single image-based study (Amon et al. 2016; Simon-Lledó et al. 2019b; Uhlenkott et al. 2022). Environmental drivers acting at different scales promote habitat complexity and modulate local habitat conditions (Simon-Lledó et al. 2020). These environmental drivers include particulate organic carbon (POC) flux in an approximate latitudinal gradient (Pennington et al. 2006; Volz et al. 2018), depth along a longitudinal gradient, and a distinct seafloor topography in the form of sediment plains, nodule fields, and seamounts (Wedding et al. 2013) that are inhabited by different megafauna communities (e.g., Simon-Lledó et al. 2019a; Cuvelier et al. 2020; Durden et al. 2021). Such differences have also been observed within and beyond the CCZ region for fish and invertebrate scavengers, a highly mobile group of organisms capable of covering considerable distances, although observed differences were related to abundance changes rather than shifts in the taxonomic composition (Drazen et al. 2021). Megafaunal organisms form an important component of the benthic deep-sea community and their bioturbation activities and processing of phytodetritus play a major role in ecosystem functioning of the abyssal community (Smith et al. 2008).
Megafauna in marine environments is defined as all organisms visible on photographs (Bluhm 1994), accounting for individuals of a size of ≥1 cm (Dunlop et al. 2015; Moleón et al. 2020). In benthic ecological studies, megafauna is often used as a loose term for benthic species living either in the upper sediment layer, on the seafloor, or directly above the seafloor in the water column (e.g., Amon et al. 2016; Simon-Lledó et al. 2019a; Schoening et al. 2020). These organisms include vertebrates and invertebrates, which can be assigned to functional groups according to their locomotion mode (e.g., sessile, hemi-sessile and mobile), dietary composition (e.g., suspension feeder, detrivore, scavenger, predator), dispersal mode (e.g., planktonic larvae or breeding), ability to colonize new or disturbed habitats (pioneering, intermediate or climax), and capability of creating and providing a habitat for associated taxa (Tavares et al. 2019; Kuhnz et al. 2020).
For the CCZ region, a total of 2,928 taxa from the water surface to the deep sea seafloor are known from ten datasets available in OBIS, including 1,440 which have been identified to species level (www.obis.org; accessed on 23.06.2022). Megafauna taxa have been observed across distribution ranges of several hundreds of kilometers (e.g., Amon et al. 2016; Cuvelier et al. 2020; Drazen et al. 2021), with a gradual change in the species composition correlating to distances between study areas (Simon-Lledó et al. 2020). Nevertheless, many rare taxa are observed, occurring only once or less than ten times within a specific study area (Amon et al. 2016; Durden et al. 2021).
This review focuses on case studies conducted on the benthic megafauna of the area BGR-E and puts these into the context of reviewed observations obtained across the entire CCZ. We will review important influences on megafauna such as bathymetry, POC flux and nodule presence, that have been shown to influence distribution pattern, diversity and community structure of megafaunal organisms at different scales. We present conclusions regarding these gradients and the levels to which these factors have an influence on the megafauna. In addition, we discuss the potential impacts of mining activities on megafaunal organisms in different habitats and include ideas for mitigation strategies to reduce such impacts.
Study area and sampling campaigns
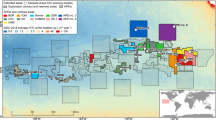
The area BGR-E is located at the eastern margin of the CCZ, being characterized by comparably high POC-flux and an average water depth of 4200 m (Kuhn and Rühlemann 2021). Bathymetrically, BGR-E is interspersed with seamounts (Fig. 1). Furthermore, north-to-southward-oriented hill and trough structures occur, especially in the western parts of BGR-E (Fig. 1).
A map of the CCZ marking the exploration areas issued by the International Seabed Authority (straight black lines) and Areas of Particular Environmental Interest (APEIs; dashed black lines) (International Seabed Authority 2020, 2021). The background map reflects low-resolution bathymetry in the area (GEBCO 2014). The area BGR-E (red lines) is shown in higher resolution in the lower, right-hand map
The megafauna inhabiting BGR-E has been investigated during ten cruises conducted between 2010 and 2021. The most commonly used analysis method was non-invasive imagery identification, by using either towed camera systems (eight cruises) or autonomous underwater vehicles (AUV; two cruises) for photographic surveys (Table 1).
The advantages of photographic surveys are the non-invasive approach (Schoening et al. 2020) and the potentially large seafloor coverage. Except for a short-term disturbance due to the light and the passage of the camera platform, the environment is not altered. Identification of megafauna specimens from images is usually not possible on species level (Horton et al. 2021). Nevertheless, megafauna observations have been referred to as morphotypes in the BGR-E area (Uhlenkott et al. 2022, under review), a common strategy in the CCZ (e.g., Amon et al. 2016; Simon-Lledó et al. 2020; Durden et al. 2021).
In addition to this non-invasive approach, selective sampling of specimens was conducted with remotely operated vehicles (ROVs) in 2015, 2019, and 2021 (Table 1). Further megafaunal specimens were sampled using a boxcore, an epibenthic sledge, a multicorer, and a chain bag dredge. Baited traps were deployed to attract and sample scavengers in 2015, 2019, and 2021, and a lander equipped with bait and a camera collected video imagery of the scavenger communities within BGR-E in 2018.
Physical sampling is necessary for species identification, as specimens can then be morphologically characterized and described in detail. This can be complemented by molecular methods to support morphological identification, especially by using DNA-barcodes (e.g., Kersken et al. 2018b; Christodoulou et al. 2020). Further methods, such as proteomic fingerprinting and food-web analyses with stable isotopes, among other approaches, can only be conducted with actual specimens. In this context, the selective sampling with ROVs is most suitable, since it offers the possibility to observe specimens in their natural habitat, thus expressing their original habitus prior to the collection.
The megafauna community of the BGR-E area
The BGR-E area exhibits megafauna abundances and diversity comparable to neighbouring areas in the eastern CCZ (e.g., Amon et al. 2016; Simon-Lledó et al. 2019a, 2020; Cuvelier et al. 2020; De Smet et al. 2021) and elevated densities compared to more western areas (e.g., Simon-Lledó et al. 2020; Durden et al. 2021). Among the mobile taxa, especially Echinodermata are very abundant (Vanreusel et al. 2016), with high numbers of different morphotypes of the classes Ophiuroidea, Asteroidea and Holothuroidea (Uhlenkott et al. 2022) (Fig. 2). The most common sessile taxa contributing a high number of morphotypes are Porifera and Cnidaria (Vanreusel et al. 2016) (Fig. 2). Especially the taxa Alcyonacea and Actiniaria are prominent, with a large number of different morphotypes being observed in the BGR-E area (Uhlenkott et al. 2022). Further megafauna taxa such as Arthropoda, Polychaeta, and Mollusca, as well as different fish species and Xenophyophora (large single-celled organisms), were observed and increase the diversity in this area.
Examples of megafauna invertebrate taxa observed in the BGR-E area on still images obtained with towed camera systems, a Abyssoprimnoa gemina sp. inc. (ALC_008); b Bathygorgia profunda sp. inc. (ALC_004); c Columnella sp. indet. mtp-BRY_003; d Actiniaria mtp-ACT_067; e Actiniaria mtp-ACT_023; f Corallimorphus sp. indet. mtp-COR_001; g Antipatharia mtp-ANT_006; h Abyssopathes lyra sp. inc. (ANT_002); i Docosaccus maculatus sp. inc. (HEX_015); j Hyocrinidae mtp-CRI_030; k Urechinus sp. indet. mtp-URC_009; l Holascus taraxum sp. inc. (HEX_018); m Peniagone leander sp. inc. (HOL_028); n Ophiosphalma glabrum sp. inc. (OPH_010); o Caulophacus variens sp. inc. (POR_050); p Hyphalaster sp. indet. mtp-AST_007; q Freyella sp. indet. mtp-AST_001; r Munidopsis sp. indet mtp-DEC_007; scale bars refer to 5 cm
Uhlenkott et al. (2022) and Uhlenkott et al. (under review) identified 250 morphotypes from 10.509 specimens in 8292 seafloor images, which coincides with the number of morphotypes or species richness reported from surrounding contractor areas (e.g., Amon et al. 2016; Simon-Lledó et al. 2019a). However, many megafauna morphotypes can be regarded as rare (Amon et al. 2016; Durden et al. 2021; Uhlenkott et al. 2022, under review), and a high number of yet undetected morphotypes have to be expected. According to extrapolation analyses computed for the BGR-E area, the total number of morphotypes might vary between 278 and up to 350 (Table 2). Comparing the expected number of morphotypes of areas covered with nodules and rock outcrop, rarefaction curves suggest a similar increase in the number of morphotypes with greater numbers of investigated specimens (Fig. 3). This increase appears to be slightly lower in areas without nodule coverage (Fig. 3). However, the increase is higher when all habitats are combined, and a plateau of encountered morphotypes has not yet been reached when including more than 10,000 analysed specimens (Fig. 3).
Rarefaction curves based on specimens obtained from 5969 photographic images from the BGR-E area, being assigned to 250 morphotypes for all available specimens as well as for specimens observed in images depicting a nodule free area, coverage with small nodules, large nodules, and rock outcrop (Uhlenkott et al. 2022, under review). Rarefaction curves were computed with the function rarecurve from the R-package vegan (Oksanen et al. 2019)
Directly comparing the BGR-E area to the adjacent contract areas of Global Sea Mineral Resources (GSR, Belgium) and Institut Français de Recherche pour l'Exploitation de la Mer (IFREMER, France), the observed megafauna density is lowest in the BGR-E area despite having the highest predicted POC flux (Vanreusel et al. 2016). POC flux is an environmental variable that is often equated to food availability, which usually is a limiting factor in the deep sea (Smith et al. 2008) and decreases with increasing water depth (Clark et al. 2010; Ostmann and Martínez Arbizu 2018). On a larger scale, this can indeed be hypothesized to drive differences between the low megafauna densities of 0.04-0.06 individuals per m2 observed in the abyssal plains of the western “Areas of Particular Environmental Interest” (APEIs; Fig. 1) (Durden et al. 2021) in comparison to the mean density of 0.34 individuals per m2 observed in the BGR-E area (Uhlenkott et al. under review). Comparing these three western APEIs, Durden et al. (2021) recognized changes in the composition of sponges, with more filter-feeding morphotypes in areas of higher POC flux and more carnivorous Demospongiae in areas of lower POC flux. Based on these observations, we (1) hypothesize that large differences in POC flux across the CCZ may cause differences in megafauna densities—with lower densities in lower POC flux western areas and with higher densities in higher POC flux eastern areas, and (2) hypothesize that comparably small changes in POC flux such as within the eastern CCZ area may cause differences in community composition.
On a local scale, the presence, density, spatial coverage, and size of manganese nodules are the most prominent factors that create habitat patchiness on the abyssal plains of the CCZ (Volz et al. 2018; Simon-Lledó et al. 2020). Generally, nodules are a key hard substratum for settlement (Amon et al. 2016; Simon-Lledó et al. 2019c). Furthermore, the relationship between nodules and the sediment, for example how far (large) nodules stick out of the sediment, has an effect on sediment characteristics and micro-scale currents in between the nodules (De Smet et al. 2017), also leading to an increase in habitat heterogeneity (Ramirez-Llodra et al. 2011; Tilot et al. 2018).
In BGR-E, areas covered with nodules provide a similar taxonomic richness of 32–34 taxa per 100 individuals compared to areas covered with rock outcrop (29±4 taxa per 100 individuals) and sediment plain sites devoid of nodules (29±3 taxa per 100 individuals) (Uhlenkott et al. under review). However, density was the highest in rock-covered areas with 1.4 individuals per m2 and lowest in nodule-free areas with only 0.2 individuals per m2, while density amounted to 0.3–0.4 individuals per m2 in nodule-covered sites (Uhlenkott et al. under review). In the CCZ, megafauna density has been observed to be in general 2–3 times higher in nodule fields compared to adjacent nodule-free sites within the same contractor area (Vanreusel et al. 2016). An explanation for the higher densities in nodule areas might be the co-occurrence of sediment specialists and nodule fauna due to the increase in habitat heterogeneity (Vanreusel et al. 2016; Simon-Lledó et al. 2019b). The absence of the coral taxa Alcyonacea and Antipatharia in nodule-free areas as observed in 2015 by Vanreusel et al. (2016) could not be confirmed in the vicinity of bathymetric elevations in the BGR-E area (Uhlenkott et al. under review). In contrast, two alcyonacean morphotypes identified as Callozostron bayeri Cairns, 2015 and a morphotype of the family Primnoidae were two of the three only morphotypes recognized as generalists for areas covered with rock outcrop, nodules of varying sizes and soft substratum (Uhlenkott et al. under reveiew) (Table 3).
Density was also described to be especially variable in nodule areas compared to seamount areas (Cuvelier et al. 2020) and direct comparisons between rock-covered sites and nodule areas revealed that the density is 2 times higher in areas characterized by rock outcrop (Uhlenkott et al. under review). Regarding the megafauna community at seamounts, the composition varies distinctly from hard- or soft-substrate megafauna in the surrounding abyssal plains (Cuvelier et al. 2020; Durden et al. 2021). In a distribution modelling approach applied in the BGR-E area, seamount areas could clearly be differentiated from plain areas based on the predicted megafauna community (Uhlenkott et al. 2022). Comparing seamounts from the BGR-E area to the most northern GSR contract area, differences in megafauna occurring on seamounts were apparent, while the nodule fauna in both areas was estimated to be more homogenous (Cuvelier et al. 2020). Both contract areas showed a clear distinction from the megafauna composition in APEI-3 (Cuvelier et al. 2020).
Case studies on different megafaunal taxa
Not all taxa occurring in the BGR-E area have been investigated in similar detail and sampling effort. However, focusing on more common and “model” organisms such as Ophiuroidea (Christodoulou et al. 2020) or the macrofauna taxa Polychaeta and Isopoda (Janssen et al. 2019; Brix et al. 2020) does not necessarily mean that they represent indicator species or play a key role within the community. It is a bias based on the fact that scientific expertise is usually not available for all occurring taxa, and hence, studies are conducted on the taxa that can be most efficiently investigated. All in all, the deep sea still remains a largely under-investigated environment (Ramirez-Llodra et al. 2010), although the CCZ and the BGR-E area are among the best studied deep-sea areas.
Porifera
Several glass sponges have been observed in the BGR-E area and investigated in detail. The species Hyalonema (Prionema) breviradix Kersken, Janussen & Martínez Arbizu, 2018 was recently described from a holotype obtained in the BGR-E area (Kersken et al. 2018a). As described for further species of the genus Hyalonema that are observed in the BGR-E area, it grows on sediment and is not attached to hard substrates (Kersken et al. 2018a). Hyalonema (Prionema) breviradix has an uncommon, disc-shaped habitus and only rudimentary anchoring basalia, which is the reason why the sponge body appears to grow on the sediment surface (Kersken et al. 2018a). The recently described species Caulophacus (Caulophacus) wilsoni uses polymetallic nodules as hard substratum to grow on; it is stalked, with a rigid stalk and a soft discoidal head (Kersken et al. 2019).
Porifera is one of the most diverse and abundant group occurring on hard and soft substrates throughout the CCZ (Foell and Pawson 1986; Kersken et al. 2018a, 2019), which could not be observed in nodule areas of the Indian Ocean (Sharma and Rao 1992). Two major groups dominate the nodule fields in the CCZ, carnivorous Demospongiae and filter-feeding Hexactinellida, either attached to hard substrates or on the sediment (Kersken et al. 2018a). Within the group Demospongiae, the family Cladorhizidae dominates the species richness and abundance (Kersken et al. 2018a). They are structuring species that offer microhabitats for associated taxa living on them, such as suspension feeders (Beaulieu 2001; Stratmann et al. 2021). Especially in sedimented areas, Porifera are the dominant group outnumbering cnidarians (Tilot et al. 2018), which is probably related to the ability of some species to attach themselves directly to the sediment (Kersken et al. 2019).
Ophiuroidea
An investigation of Ophiuroidea in the CCZ and the Peru Basin based on DNA-barcoding revealed a total of 43 species (Christodoulou et al. 2020). The most common taxa across all areas were Ophioleucidae sp. (species 29), Amphioplus daleus (Lyman, 1879) (species 2) and Ophiosphalma glabrum Lütken & Mortensen 1899 (species 3) (Christodoulou et al. 2020). Of all 43 species identified, 24 occurred in the BGR-E area, including six exclusively sampled taxa (Christodoulou et al. 2020), thereby representing the highest species richness and number of exclusively observed taxa of all investigated areas. However, the highest number of samples and 40% of all sampled specimens were obtained from the BGR-E area (Christodoulou et al. 2020), which explains the highest number of identified species in this area. Nevertheless, although the slope of the rarefaction curve seemed to flatten slightly for the BGR-E area, no plateau has yet been reached. Extrapolation based on the Chao1 Index suggests that a total number of 57 ophiuroid species can be expected (Christodoulou et al. 2020).
Comparing the ophiuroid assemblages of the different contract areas based on relative abundances, the BGR-E area is most similar to the UK-1 contract area, which is located to the northeast of it (Christodoulou et al. 2020). Most pronounced differences were observed between contract areas and APEI-3 at the very north of the CCZ (Christodoulou et al. 2020). Overall, the biodiversity of Ophiuroidea was considerably higher than expected, especially in relation to the low POC flux in the CCZ (Christodoulou et al. 2019, 2020).
Bryozoa
Colonies of bryozoans are often observed in imagery surveys of megafauna in the CCZ, including the BGR-E area (Amon et al. 2017a; Simon-Lledó et al. 2019a; Uhlenkott et al. 2022), and are also commonly obtained as epifauna in boxcore samples. After a mobile larval stadium, individuals of this taxon settle on hard substratum and form sessile colonies (Hurlbut 1991). Therefore, a pioneer zooid settles in a suitable habitat, being the first member of a colony formed of clones (Brusca and Brusca 1990). In the BGR-E area, 72 different molecular operational units (MOTUs), i.e., species equivalents, were obtained from boxcore samples, of which 23 MOTUs were only observed once (Focke Weerts & Pedro Martínez Arbizu, pers. comm.). These first preliminary results provide a glance of the high species richness and abundance of this megafauna group in the BGR-E area and its contribution to megafauna communities within contract areas of the CCZ.
Cnidaria
Cnidaria are an abundant and diverse group in the CCZ, contributing significantly to the megafauna biodiversity obtained from imagery studies (Foell and Pawson 1986; Amon et al. 2016) as well as sampling and genetic analysis (Dahlgren et al. 2016). They occur commonly attached to hard substrates, either on seamounts or in nodules fields (Dahlgren et al. 2016; Cuvelier et al. 2020). In sedimented areas, the diversity and abundance of certain groups are reduced (Tilot et al. 2018). Within nodule fields especially the groups Antipatharia and Alcyonacea are among the most abundant and diverse taxa (Vanreusel et al. 2016; Stratmann et al. 2018b). For example, the alcyonacean Abyssoprimnoa gemina was the fourth-most abundant taxon in the UK-1 and an additional site located 250 km eastern in the eastern CCZ (Amon et al. 2016). In the BGR-E area, two alceonacean corals could be identified as generalist morphotypes (Uhlenkott et al. under review).
Xenophyophora
Xenophyophora are single-cell organisms and, hence, often excluded in surveys of the metazoan megafauna. Still, this taxon displays a high diversity and often highest abundances of megafauna in various imagery studies within the CCZ (Kamenskaya et al. 2013; Simon-Lledó et al. 2019d; Durden et al. 2021). In the western CCZ, at least 22 morphotypes were observed (Gooday et al. 2020), whereas 36 morphotypes were registered from two contract areas in the eastern CCZ (Gooday et al. 2017), dominating the megafauna with highest observed abundances in both studies. They occur on sediment plains and within nodules fields, many of them directly attached to nodules (Gooday et al. 2020). Xenophyophora are suggested to be a trophic link between bacteria and larger fauna (Amon et al. 2016), although contributing little to the total biomass of the megafauna in general (Gooday et al. 2020; Durden et al. 2021). Diversity and density of Xenophyophora are potentially influenced by geomorphological variations regulating local bottom water flow as well as by the presence and absence of nodules (Simon-Lledó et al. 2019a; Durden et al. 2021).
Scavengers
In the BGR-E area, scavenger biodiversity was investigated based on 10 in situ baited camera experiments in 2018 (Harbour et al. 2020) and compared with further data from baited traps and video transects of the westerns CCZ and further areas throughout the Pacific Ocean (Drazen et al. 2021). A total of twelve scavenging taxa could be observed, the most abundant being the rattails Coryphaenoides spp. Gunnerus, 1765, followed by the eelpout Pachycara nazca Anderson & Bluhm, 1997 and the shrimp Hymenopenaeus nereus (Faxon, 1893). Coryphaenoides spp. was also responsible for the majority of bait consumption (Harbour et al. 2020).
Leitner et al. (2017) suggested that high nodule abundance could lead to an increased abundance of scavengers, potentially due to higher prey abundance in nodule areas. All sites investigated in the BGR-E area had similar nodule abundances but differing predominant nodule size, yet no significant differences in scavenger abundance could be detected as a function of nodule size (Harbour et al. 2020). However, the scavenger community differed significantly with that of the neighbouring contract areas UK-1 and OMS (Harbour et al. 2020). Especially seamounts and abyssal plains showed significant variations of the scavenger community, contributing to a regional diversity (Drazen et al. 2021). Species composition of scavenging amphipods, within the BGR-E area was similar to other areas within the CCZ, but differed from the more southern Pacific Peru Basin (Patel et al. 2020).
Megafauna in the context of deep-sea mining
The deep-sea environment is known to be highly sensitive to physical disturbance, and various tests of mining components and disturbance experiments have shown long-term influences of mining-like disturbances on the ecosystem (Jones et al. 2017). Polymetallic nodules provide an important habitat for organisms of all size classes (Thiel et al. 1993; Vanreusel et al. 2016; Purser et al. 2016), and large-scale removal forms a direct threat to these highly diverse, low biomass deep-sea communities. In addition, depending on the type of machinery used, mining activities are likely to provoke the re-suspension of a large amount of sediment in the form of a sediment plume, which will spread out into the surrounding, unmined areas and may additionally harm organisms that were not mechanically affected by mining processes (Fig. 4). Plumes with greatly increased particle concentrations may especially affect filter-feeders, and settling sediment out of the plume will cover the nodules, sediments, and organisms of the seafloor, thus affecting all faunal classes. Suspension of sediments and nodule debris may also enhance trace metal mobilization, which when bioavailable could have adverse ecotoxicological consequences (Jankowski and Zielke 2001; Hauton et al. 2017; Brown et al. 2017; Schmidt et al. 2022).
Modified graphical representation of PRZs and IRZs as set out in the ISA mining code and in recommendations presented in Jones et al. (2020). + refers to areas already defined under the Mining Code. * refers to additional areas recommended by Jones et al. (2020). The relative size of each area is not to scale. The pink and blue outlined IRZs and PRZs indicate the intended implementation of this concept by two contractors according to Hao et al. (2020) for COMRA and for the BGR-E area (BGR unpublished information)
In this context, the APEIs have been proposed as protected areas which should cover the range of habitats and biological variations present within the CCZ (Wedding et al. 2013). The ISA adopted this framework in a reduced form of initially nine APEIs, each with a size of 400×400 km (160,000 km2), and adopted four additional APEIs of varying sizes in 2021 (Fig. 1). Each of the initial APEI consists of a core area of 200×200 km and a buffer zone of 100 km to protect the core from any anthropogenic, mining related impact (Wedding et al. 2013). However, the initial APEIs are all positioned on the margins of the CCZ and have been strongly criticized as they do not effectively represent all habitats and show a limited connectivity to the contract areas (Taboada et al. 2018; McQuaid et al. 2020; Washburn et al. 2021; Jones et al. 2021). The lack of representation has been related to differences in depth, the number of seamounts, nodule density and coverage and seafloor POC flux within APEIs compared to adjacent contract areas or CCZ sub-regions (McQuaid et al. 2020; Washburn et al. 2021). Hence, the ISA has recently integrated scientific recommendations (e.g., Vanreusel et al. 2016; Simon-Lledó et al. 2020; Bonifácio et al. 2020; Christodoulou et al. 2020; Brix et al. 2020) and established four additional APEIs, of which one (APEI-13) is positioned within the core manganese nodule area of the CCZ (Fig. 1).
Within contract areas, contractors are required to designate Impact Reference Zones (IRZs), where potential future mining activities will take place, and Preservation Reference Zones (PRZs), which are outside the mined areas and beyond the Impact Zone (e.g., the area affected by sediment plume dispersal; Fig. 4). Officially, these zones have been established in order to assess the impact caused by mining activities against a pristine control site (Jones et al. 2020). In the BGR-E area, one prospective mining area has been assigned together with a potential preservation reference zone in 2013 in order to carry out spatial and temporal studies of biodiversity in both areas (see schematic in Fig. 4). Similar to the approach applied for the APEIs (Wedding et al. 2013), the PRZ should be located as close as possible to the IRZ to maintain representativity, but far enough to not be affected by any disturbance from it. Beyond that, it has been suggested that additional preservation, respectively conservation, areas in a similar design as the PRZs could be chosen to include a variety of different habitats, e.g., seamounts, nodule-covered and nodule-free sediment plains, in order to additionally account for potential mining impacts on these habitats (Jones et al. 2020). However, studies in the BGR-E area over several years have shown that the geochemical seafloor conditions and the communities in the PRZ are much more diverse and spatially variable than they are in the IRZ. Based on available distribution data and modelling approaches, Uhlenkott et al. (2020) and Uhlenkott et al. (2022) have shown that meiofauna abundances and megafauna communities vary distinctly between the designated PRZ and IRZ. This prompts the designation of a more suitable PRZ in terms of its function as a control site, whereas the currently designated PRZ will have a good protective function.
In an expert elicitation to determine which ecosystem components are the most relevant variables for monitoring and conservation in the deep sea, megafauna appeared as one of the highest-ranked variables (Danovaro et al. 2020a). Despite discussions that small animals also need to be considered as they constitute an important part of the benthic ecosystem (Danovaro et al. 2020b; Ingels et al. 2020), megafauna has the major advantage that it can be observed based on imagery, which is a fast, area-wide, non-invasive monitoring technique that does not alter the environment (Schoening et al. 2020). Additionally, the longevity of sessile megafauna allows to revisit individual organisms for long-term investigations (Schoening et al. 2020).
In the BGR-E area, the effects of test mining actions have not yet been profoundly investigated, but will be further examined in the next years following a pre-prototype collector test that was conducted and monitored in spring 2021 (Vink and shipboard scientific party, in press). Furthermore, a number of small-scale disturbance experiments simulating potential impacts of nodule mining have been conducted to monitor and evaluate anthropogenic impacts in the BGR-E area and in other areas (e.g., Foell et al. 1990; Fukushima 1995; Sharma 2000; Jones et al. 2017; Purkiani et al. 2021).
One of the largest and most studied disturbance experiments was the “DISturbance and reCOLonization experiment” (DISCOL) in the nodule-covered Peru Basin, in 1989 (Thiel et al. 2001). The disturbance showed significant effects, such as a modelled reduction of carbon flow in the food web and variations in the recovery of organisms with different feeding types, with suspension and filter feeders showing the lowest recovery rates (Stratmann et al. 2018a). Investigations of the same area based on AUV photo mosaics conducted 26 years after the impact confirmed the significant reduction in megafaunal suspension feeders, whereas the densities of deposit feeders as well as scavengers and predators had recovered to pre-impact values (Simon-Lledó et al. 2019c).
These findings can be confirmed by investigations of Holothuroidea as an example for mobile taxa, which recovered within 26 years in terms of their community composition, abundance and respiration rates (Stratmann et al. 2018b). Similarly, fish density recovered after 26 years, but the density of the dominant species Ipnops meadi Nielsen, 1966 remained lower in the disturbed areas (Drazen et al. 2019). Investigations of the scavenging fauna using baited traps revealed no significant differences comparing the scavenging fauna inside the impact area of the “plough-harrow” with undisturbed reference sites 6 months and 3 years after the impact (Drazen et al. 2019). Indirect impacts induced by the increased sedimentation of the created sediment plume showed an immediate density reduction followed by an increase with higher densities compared to reference areas of the pre-impact studies of certain taxa (Bluhm 2001).
Other disturbance experiments were conducted using the Deep-Sea Sediment Resuspension System (DSSRS) developed by NOAA in different areas in the CCZ (Jones et al. 2017). In the contract area of the Interoceanmetal Joint Organisation (IOM), megafauna in the tracks showed a significant decrease in taxonomic richness and abundance with a slow recovery after the disturbance (Radziejewska and Stoyanova 2000). Previously observed differences between areas covered with nodules and those devoid of nodules remained after the disturbance event in both areas, but the sediment plains showed a faster recovery in terms of species richness and densities compared to nodule areas (Radziejewska and Stoyanova 2000). The Japan Deep-sea Impact Experiment (JET) was conducted in the western part of the CCZ and showed a reduction in density of motile fauna and deposit feeders in the indirect disturbed areas affected by sediment plumes compared to the undisturbed ones, but no significant difference and reduction in sessile megafauna could be observed two years after the disturbance event (Fukushima et al. 2000).
Vanreusel et al. (2016) used a different approach, investigating the impacts of previous scientific sampling disturbances on the megafauna community. Within the tracks of sampling gear such as dredge or epibenthic sledge, all hard substratum was removed and almost no sessile megafauna could be observed. Mobile megafauna was significantly reduced in abundance even after 20 and 37 years, respectively (Vanreusel et al. 2016). This observation is not limited to megafauna, as density and diversity of the meiofauna taxon Nematoda were also significantly reduced 26 years after experimental dredging (Miljutin et al. 2011). Comparing the varying results, the extent of recovery is presumably closely linked to the extent of the disturbance (Thiel et al. 2001) as well as to the sensitivity of the different taxa towards the mining impact (Simon-Lledó et al. 2019c).
Current knowledge gaps include the assessment of temporal (interannual) variability, seasonality and awareness that more samples and larger sampling areas are needed, especially when many rare morphotypes are present (Bluhm 2001; Durden et al. 2021). The sparse distribution of such rare taxa complicates impact assessments compared to shallow water assessments, where taxa often occur in more aggregated patterns and higher densities (Schoening et al. 2020). Nodule areas show a high habitat heterogeneity related to environmental drivers acting at different scales in addition to biological interactions, which are not yet fully understood (Simon-Lledó et al. 2020). Nodule-attached fauna can make up to 60–70% of the total megafauna density within nodule fields, and so the removal will have severe long-term effects for these taxa in the mined areas (Simon-Lledó et al. 2019c). Some nodule obligate taxa will probably never recover (Vanreusel et al. 2016). The implementation of mitigation strategies for compensation of habitat loss has often been discussed in a scientific context (Vanreusel et al. 2016; Gollner et al. 2017, 2022; Cuvelier et al. 2018) and is now also being adopted by the ISA in its regulations for Environmental Impact Assessment and monitoring (International Seabed Authority 2021).
Conclusion
The CCZ is the largest and most economically interesting area targeted for the mining of deep-sea polymetallic nodules on earth. The BGR-E area is positioned in the very east of the CCZ, is shallower than more western areas, and exhibits a relatively high POC flux. In general, POC flux is connected to elevated megafauna densities in the food-limited deep sea (Smith et al. 2008). This could be validated through the comparison of the BGR-E area to the APEIs at the western edge of the CCZ (Durden et al. 2021). Within the eastern CCZ, however, lower megafauna densities are found in the BGR-E area than in the more westerly IFREMER and GSR contract areas, despite higher predicted POC flux input (Vanreusel et al. 2016). Hence, megafauna density is not exclusively controlled by food availability.
Within the BGR-E area, density has been described to be highest on rock outcrops (Uhlenkott et al. under review), which is usually of volcanic origin and, hence, observed at seamounts and abyssal hill sites. In contrast, Cuvelier et al. (2020) observed higher densities in nodule fields compared to seamount sites in the BGR-E area. Nevertheless, both studies show that the megafaunal community observed on rock outcrop, respectively seamount, sites is different compared to that of the abyssal plains (Cuvelier et al. 2018; Uhlenkott et al. under review). Taxonomic richness and biodiversity are especially high in the nodule areas of the BGR-E area (Uhlenkott et al. under review), which might be related to the number of available niches and an enhanced food supply in this heterogenous habitat. Habitat heterogeneity may therefore be responsible for either increased biodiversity and densities or both, depending on the investigated habitat or landscape.
Detailed studies of specific megafauna groups in the BGR-E area have focused on the taxa Ophiuroidea (Christodoulou et al. 2019, 2020) and Porifera (Kersken et al. 2018a, b, 2019) as well as on the scavenging community (Harbour et al. 2020; Patel et al. 2020). This selection of studied functional groups is merely based on the expertise of the investigating experts. Therefore, many taxa and functional groups still require detailed investigation to assess key functional groups and taxa despite the fact that the BGR-E area is a comparably well-studied deep-sea area.
The benthic megafaunal community of the CCZ will be threatened by the impacts of deep-sea mining activities. In contrast to the majority of other contract areas in the CCZ, impact and preservation reference zones for a prospective mining area have been designated and analysed in the BGR-E area since 2013. Faunal and geochemical analyses increasingly show that the PRZ does not fulfil its function as a control site for the determination of mining-related impacts. It could, however, be maintained as a “preservation area” sensu stricto, whereas a more suitable PRZ will need to be defined for the assessment of impacts. Additional “conservation areas” within contractor areas, integrating differences in the faunal community as a whole, would be beneficial additions.
The original APEIs and the BGR-E area show only little overlap in their habitats and benthic megafaunal communities, showing that they are not suitable for the protection of megafaunal biodiversity from the BGR-E area. However, the new APEIs potentially better represent the habitats present in the BGR-E area. Still, future studies in the new APEIs need to be conducted to validate their representability and protective function.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files including references to their original source.
References
Amon DJ, Ziegler AF, Dahlgren TG et al. (2016) Insights into the abundance and diversity of abyssal megafauna in a polymetallic-nodule region in the eastern Clarion-Clipperton Zone. Sci Rep 6:30492. https://doi.org/10.1038/srep30492
Amon DJ, Ziegler AF, Drazen JC et al. (2017a) Megafauna of the UKSRL exploration contract area and eastern Clarion-Clipperton Zone in the Pacific Ocean: Annelida, Arthropoda, Bryozoa, Chordata, Ctenophora, Mollusca. Biodivers Data J 5:e14598. https://doi.org/10.3897/BDJ.5.e14598
Amon D, Ziegler A, Kremenetskaia A, et al. (2017b) Megafauna of the UKSRL exploration contract area and eastern Clarion-Clipperton Zone in the Pacific Ocean: Echinodermata. Biodivers Data J 5:e11794. https://doi.org/10.3897/BDJ.5.e11794
Beaulieu SE (2001) Life on glass houses: sponge stalk communities in the deep sea. Mar Biol 138:803–817. https://doi.org/10.1007/s002270000500
Bluhm H (1994) Monitoring megabenthic communities in abyssal manganese nodule sites of the East Pacific Ocean in association with commercial deep-sea mining. Aquat Conserv Mar Freshw Ecosyst 4:187–201. https://doi.org/10.1002/aqc.3270040302
Bluhm H (2001) Re-establishment of an abyssal megabenthic community after experimental physical disturbance of the seafloor. Deep Sea Res Part II Top Stud Oceanogr 48:3841–3868. https://doi.org/10.1016/S0967-0645(01)00070-4
Boetius A, Haeckel M (2018) Mind the seafloor. Science 359:34–36. https://doi.org/10.1126/science.aap7301
Bonifácio P, Martínez Arbizu P, Menot L (2020) Alpha and beta diversity patterns of polychaete assemblages across the nodule province of the eastern Clarion-Clipperton Fracture Zone (equatorial Pacific). Biogeosciences 17:865–886. https://doi.org/10.5194/bg-17-865-2020
Brix S, Osborn KJ, Kaiser S et al. (2020) Adult life strategy affects distribution patterns in abyssal isopods – implications for conservation in Pacific nodule areas. Biogeosciences 17:6163–6184. https://doi.org/10.5194/bg-17-6163-2020
Brown A, Wright R, Mevenkamp L, Hauton C (2017) A comparative experimental approach to ecotoxicology in shallow-water and deep-sea holothurians suggests similar behavioural responses. Aquat Toxicol 191:10–16. https://doi.org/10.1016/j.aquatox.2017.06.028
Brusca RC, Brusca GJ (1990) The lophophorate phyla: Phronoids, Ectoprocts and Branchiopods. In: Brusca RC, Brusca GJ (eds) Invertebrates. Sinauer Associates, Sunderland, USA, p 788
Christodoulou M, O’Hara T, Hugall AF et al. (2020) Unexpected high abyssal ophiuroid diversity in polymetallic nodule fields of the northeast Pacific Ocean and implications for conservation. Biogeosciences 17:1845–1876. https://doi.org/10.5194/bg-17-1845-2020
Christodoulou M, O’Hara TD, Hugall AF, Arbizu PM (2019) Dark Ophiuroid Biodiversity in a Prospective Abyssal Mine Field. Curr Biol 29:3909–3912.e3. https://doi.org/10.1016/j.cub.2019.09.012
Clark MR, Rowden AA, Schlacher T et al. (2010) The Ecology of Seamounts: Structure, Function, and Human Impacts. Annu Rev Mar Sci 2:253–278. https://doi.org/10.1146/annurev-marine-120308-081109
Cuvelier D, Gollner S, Jones DOB et al. (2018) Potential mitigation and restoration actions in ecosystems impacted by seabed mining. Front Mar Sci 5:467. https://doi.org/10.3389/fmars.2018.00467
Cuvelier D, Ribeiro PA, Ramalho SP et al. (2020) Are seamounts refuge areas for fauna from polymetallic nodule fields? Biogeosciences 17:2657–2680. https://doi.org/10.5194/bg-17-2657-2020
Dahlgren T, Wiklund H, Rabone M et al. (2016) Abyssal fauna of the UK-1 polymetallic nodule exploration area, Clarion-Clipperton Zone, central Pacific Ocean: Cnidaria. Biodivers Data J 4:e9277. https://doi.org/10.3897/BDJ.4.e9277
Danovaro R, Fanelli E, Aguzzi J et al. (2020a) Ecological variables for developing a global deep-ocean monitoring and conservation strategy. Nat Ecol Evol 4:181–192. https://doi.org/10.1038/s41559-019-1091-z
Danovaro R, Fanelli E, Aguzzi J et al. (2020b) Reply to: Ecological variables for deep-ocean monitoring must include microbiota and meiofauna for effective conservation. Nat Ecol Evol:1–2. https://doi.org/10.1038/s41559-020-01337-4
De Smet B, Pape E, Riehl T et al. (2017) The community structure of deep-sea macrofauna associated with polymetallic nodules in the Eastern Part of the Clarion-Clipperton Fracture Zone. Front Mar Sci 4. https://doi.org/10.3389/fmars.2017.00103
De Smet B, Simon-Lledó E, Mevenkamp L et al. (2021) The megafauna community from an abyssal area of interest for mining of polymetallic nodules. Deep Sea Res Part Oceanogr Res Pap 172:103530. https://doi.org/10.1016/j.dsr.2021.103530
Drazen JC, Leitner AB, Jones DOB, Simon-Lledó E (2021) Regional variation in communities of demersal fishes and scavengers across the CCZ and Pacific Ocean. Front Mar Sci 8:630616. https://doi.org/10.3389/fmars.2021.630616
Drazen JC, Leitner AB, Morningstar S et al. (2019) Observations of deep-sea fishes and mobile scavengers from the abyssal DISCOL experimental mining area. Biogeosciences 16:3133–3146. https://doi.org/10.5194/bg-16-3133-2019
Dunlop KM, Kuhnz LA, Ruhl HA et al. (2015) An evaluation of deep-sea benthic megafauna length measurements obtained with laser and stereo camera methods. Deep Sea Res Part Oceanogr Res Pap 96:38–48. https://doi.org/10.1016/j.dsr.2014.11.003
Durden JM, Putts M, Bingo S et al. (2021) Megafaunal ecology of the western Clarion Clipperton Zone. Front Mar Sci 8:671062. https://doi.org/10.3389/fmars.2021.671062
Foell E, Pawson DL (1986) Photographs of invertebrate megafauna from abyssal depths of the north-eastern equatorial Pacific Ocean. Ohio J Sci 86:61–68
Foell EJ, Thiel H, Schriever G (1990) DISCOL: A long-term, large-scale, disturbance-recolonization experiment in the Abyssal Eastern Tropical South Pacific Ocean. In: All Days. OTC, Houston, Texas, p OTC-6328-MS
Fukushima (1995) Overview “Japan Deep-Sea Impact Experiment = JET.” In: The Proceedings of the First (1995) ISOPE Ocean Mining Symposium. Tsukuba, Japan, pp 47–53
Fukushima T, Shirayama Y, Kuboki E (2000) The characteristics of deep-sea epifaunal megabenthos community two years after an artificial rapid deposition event. Publ Seto Mar Biol Labaratory 39:17–27
GEBCO (2014) The GEBCO_2014 Grid, version 20150318. In: Gen. Bathymetr. Chart Oceans. http://www.gebco.net. Accessed 2 Jul 2018
Glover AG, Wiklund H, Rabone M et al. (2016) Abyssal fauna of the UK-1 polymetallic nodule exploration claim, Clarion-Clipperton Zone, central Pacific Ocean: Echinodermata. Biodivers Data J:e7251. https://doi.org/10.3897/BDJ.4.e7251
Gollner S, Haeckel M, Janssen F et al. (2022) Restoration experiments in polymetallic nodule areas. Integr Environ Assess Manag 18:682–696. https://doi.org/10.1002/ieam.4541
Gollner S, Kaiser S, Menzel L et al. (2017) Resilience of benthic deep-sea fauna to mining activities. Mar Environ Res 129:76–101. https://doi.org/10.1016/j.marenvres.2017.04.010
Gooday AJ, Durden JM, Smith CR (2020) Giant, highly diverse protists in the abyssal Pacific: vulnerability to impacts from seabed mining and potential for recovery. Commun Integr Biol 13:189–197. https://doi.org/10.1080/19420889.2020.1843818
Gooday AJ, Holzmann M, Caulle C et al. (2017) Giant protists (xenophyophores, Foraminifera) are exceptionally diverse in parts of the abyssal eastern Pacific licensed for polymetallic nodule exploration. Biol Conserv 207:106–116. https://doi.org/10.1016/j.biocon.2017.01.006
Haeckel M, Linke P, shipboard scientific party (2021) SO268 Assessing the impacts of nodule mining on the deep-sea environment, Nodule monitoring. GEOMAR Helmholtz-Zentrum für Ozeanforschung, Kiel, Germany
Hao H, Lei W, Danyun O, et al. (2020) A preliminary evaluation of some elements for designation of preservation and impact reference zones in deep sea in the Clarion-Clipperton Zone: A case study of the China ocean mineral resources association contract area. Ocean Coast Manag 188:105135. https://doi.org/10.1016/j.ocecoaman.2020.105135
Harbour RP, Leitner AB, Rühlemann C et al. (2020) Benthic and demersal scavenger biodiversity in the Eastern end of the Clarion-Clipperton Zone – An area marked for polymetallic nodule mining. Front Mar Sci 7:458. https://doi.org/10.3389/fmars.2020.00458
Hauton C, Brown A, Thatje S et al. (2017) Identifying toxic impacts of metals potentially released during deep-sea mining—a synthesis of the challenges to quantifying risk. Front Mar Sci 4:368. https://doi.org/10.3389/fmars.2017.00368
Hein JR, Mizell K, Koschinsky A, Conrad TA (2013) Deep-ocean mineral deposits as a source of critical metals for high- and green-technology applications: Comparison with land-based resources. Ore Geol Rev 51:1–14. https://doi.org/10.1016/j.oregeorev.2012.12.001
Horton T, Marsh L, Bett BJ et al. (2021) Recommendations for the standardisation of open taxonomic nomenclature for image-based identifications. Front Mar Sci 8:620702. https://doi.org/10.3389/fmars.2021.620702
Hurlbut CJ (1991) Larval substratum selection and postsettlement mortality as determinants of the distribution of two bryozoans. J Exp Mar Biol Ecol 147:103–119. https://doi.org/10.1016/0022-0981(91)90040-4
Ingels J, Vanreusel A, Pape E et al. (2020) Ecological variables for deep-ocean monitoring must include microbiota and meiofauna for effective conservation. Nat Ecol Evol:1–3. https://doi.org/10.1038/s41559-020-01335-6
International Seabed Authority (2020) Deep seabed mineral resources. https://www.isa.org.jm/exploration-contracts/polymetallic-nodules. Accessed 10 Mar 2020
International Seabed Authority (2021) Decision of the Council of the International Seabed Authority relating to the review of the environmental management plan for the Clarion-Clipperton Zone. Kingston, Jamaica
Jankowski JA, Zielke W (2001) The mesoscale sediment transport due to technical activities in the deep sea. Deep Sea Res Part II Top Stud Oceanogr 48:3487–3521. https://doi.org/10.1016/S0967-0645(01)00054-6
Janssen A, Stuckas H, Vink A, Martìnez Arbizu P (2019) Biogeography and population structure of predominant macrofaunal taxa (Annelida and Isopoda) in abyssal polymetallic nodule fields: implications for conservation and management. Mar Biodivers 49:2641–2658. https://doi.org/10.1007/s12526-019-00997-1
Jones DOB, Ardron JA, Colaço A, Durden JM (2020) Environmental considerations for impact and preservation reference zones for deep-sea polymetallic nodule mining. Mar Policy 118:103312. https://doi.org/10.1016/j.marpol.2018.10.025
Jones DOB, Kaiser S, Sweetman AK et al. (2017) Biological responses to disturbance from simulated deep-sea polymetallic nodule mining. PLOS ONE 12:e0171750. https://doi.org/10.1371/journal.pone.0171750
Jones DOB, Simon-Lledó E, Amon DJ et al. (2021) Environment, ecology, and potential effectiveness of an area protected from deep-sea mining (Clarion Clipperton Zone, abyssal Pacific). Prog Oceanogr 197:102653. https://doi.org/10.1016/j.pocean.2021.102653
Kamenskaya OE, Melnik VF, Gooday AJ (2013) Giant protists (xenophyophores and komokiaceans) from the Clarion-Clipperton ferromanganese nodule field (eastern Pacific). Biol Bull Rev 3:388–398. https://doi.org/10.1134/S2079086413050046
Kersken D, Janussen D, Martínez Arbizu P (2018a) Deep-sea glass sponges (Hexactinellida) from polymetallic nodule fields in the Clarion-Clipperton Fracture Zone (CCFZ), northeastern Pacific: Part I – Amphidiscophora. Mar Biodivers 48:545–573. https://doi.org/10.1007/s12526-017-0727-y
Kersken D, Janussen D, Martínez Arbizu P (2019) Deep-sea glass sponges (Hexactinellida) from polymetallic nodule fields in the Clarion-Clipperton Fracture Zone (CCFZ), northeastern Pacific: Part II—Hexasterophora. Mar Biodivers 49:947–987. https://doi.org/10.1007/s12526-018-0880-y
Kersken D, Kocot K, Janussen D et al. (2018b) First insights into the phylogeny of deep-sea glass sponges (Hexactinellida) from polymetallic nodule fields in the Clarion-Clipperton Fracture Zone (CCFZ), northeastern Pacific. Hydrobiologia 811:283–293. https://doi.org/10.1007/s10750-017-3498-3
Kuhn T, Rühlemann C (2021) Exploration of polymetallic nodules and resource assessment: A case study from the German contract area in the Clarion-Clipperton Zone of the tropical Northeast Pacific. Minerals 11:618. https://doi.org/10.3390/min11060618
Kuhn T, shipboard scientific party (2015) SO240 FLUM: Low-temperature fluid circulation at seamounts and hydrothermal pits: heat flow regime, impact on biogeochemical processes, and its potential influence on the occurrence and composition of manganese nodules in the equatorial eastern Pacific. Bundesanstalt für Geowissenschaften und Rohstoffe (BGR), Hannover, Germany
Kuhn T, Wegorzewski A, Rühlemann C, Vink A (2017) Composition, formation, and occurrence of polymetallic nodules. In: Sharma R (ed) Deep-Sea Mining: Resource Potential, Technical and Environmental Considerations. Springer International Publishing, Cham, Switzerland, pp 23–63
Kuhnz LA, Ruhl HA, Huffard CL, Smith KL (2020) Benthic megafauna assemblage change over three decades in the abyss: Variations from species to functional groups. Deep Sea Res Part II Top Stud Oceanogr 173:104761. https://doi.org/10.1016/j.dsr2.2020.104761
Leitner AB, Neuheimer AB, Donlon E et al. (2017) Environmental and bathymetric influences on abyssal bait-attending communities of the Clarion Clipperton Zone. Deep Sea Res Part Oceanogr Res Pap 125:65–80. https://doi.org/10.1016/j.dsr.2017.04.017
Lodge M, Johnson D, Le Gurun G et al. (2014) Seabed mining: International Seabed Authority environmental management plan for the Clarion–Clipperton Zone. A partnership approach. Mar Policy 49:66–72. https://doi.org/10.1016/j.marpol.2014.04.006
Martínez Arbizu P, shipboard scientific party (2015) SO239 EcoResponse: Assessing the ecology, connectivity and resilience of polymetallic nodule field systems. GEOMAR Helmholtz-Zentrum für Ozeanforschung, Kiel, Germany
McQuaid KA, Attrill MJ, Clark MR et al. (2020) Using habitat classification to assess representativity of a protected area network in a large, data-poor area targeted for deep-sea mining. Front Mar Sci 7:558860. https://doi.org/10.3389/fmars.2020.558860
Miljutin DM, Miljutina MA, Martínez Arbizu P, Galéron J (2011) Deep-sea nematode assemblage has not recovered 26 years after experimental mining of polymetallic nodules (Clarion-Clipperton Fracture Zone, Tropical Eastern Pacific). Deep Sea Res Part Oceanogr Res Pap 58:885–897. https://doi.org/10.1016/j.dsr.2011.06.003
Moleón M, Sánchez-Zapata JA, Donázar JA et al. (2020) Rethinking megafauna. Proc R Soc B Biol Sci 287:20192643. https://doi.org/10.1098/rspb.2019.2643
Niner HJ, Ardron JA, Escobar EG et al. (2018) Deep-sea mining with no net loss of biodiversity—an impossible aim. Front Mar Sci 5:53. https://doi.org/10.3389/fmars.2018.00053
Oksanen J, Blanchet FG, Friendly M, et al. (2019) vegan: Community ecology package
Ostmann A, Martínez Arbizu P (2018) Predictive models using randomForest regression for distribution patterns of meiofauna in Icelandic waters. Mar Biodivers 48:719–735. https://doi.org/10.1007/s12526-018-0882-9
Patel T, Robert H, D’Udekem D’Acoz C et al. (2020) Biogeography and community structure of abyssal scavenging Amphipoda (Crustacea) in the Pacific Ocean. Biogeosciences 17:2731–2744. https://doi.org/10.5194/bg-17-2731-2020
Pennington JT, Mahoney KL, Kuwahara VS et al. (2006) Primary production in the eastern tropical Pacific: a review. Prog Oceanogr 69:285–317. https://doi.org/10.1016/j.pocean.2006.03.012
Purkiani K, Gillard B, Paul A et al. (2021) Numerical simulation of deep-sea sediment transport induced by a dredge experiment in the northeastern Pacific Ocean. Front Mar Sci 8:719463. https://doi.org/10.3389/fmars.2021.719463
Purser A, Marcon Y, Hoving H-JT et al. (2016) Association of deep-sea incirrate octopods with manganese crusts and nodule fields in the Pacific Ocean. Curr Biol 26:R1268–R1269. https://doi.org/10.1016/j.cub.2016.10.052
Radziejewska T, Stoyanova V (2000) Abyssal epibenthic megafauna of the Clarion-Clipperton area (NE Pacific): Changes in time and space versus anthropogenic environmental disturbance. Oceanol Stud 29:83–101.
Ramirez-Llodra E, Brandt A, Danovaro R et al. (2010) Deep, diverse and definitely different: unique attributes of the world’s largest ecosystem. Biogeosciences 7:2851–2899. https://doi.org/10.5194/bg-7-2851-2010
Ramirez-Llodra E, Tyler PA, Baker MC et al. (2011) Man and the last great wilderness: human impact on the deep sea. PLoS ONE 6:e22588. https://doi.org/10.1371/journal.pone.0022588
Rühlemann C, shipboard scientific party (2010) SO205 MANGAN. Bundesanstalt für Geowissenschaften und Rohstoffe (BGR), Hannover, Germany
Rühlemann C, shipboard scientific party (2012) BIONOD Volume 1: German License Area. Bundesanstalt für Geowissenschaften und Rohstoffe (BGR), Hannover, Germany
Rühlemann C, shipboard scientific party (2014) MANGAN 2013. Bundesanstalt für Geowissenschaften und Rohstoffe (BGR), Hannover, Germany
Rühlemann C, shipboard scientific party (2015) MANGAN 2014. Bundesanstalt für Geowissenschaften und Rohstoffe (BGR), Hannover, Germany
Rühlemann C, shipboard scientific party (2017) MANGAN 2016. Bundesanstalt für Geowissenschaften und Rohstoffe (BGR), Hannover, Germany
Rühlemann C, shipboard scientific party (2019) MANGAN 2018. Bundesanstalt für Geowissenschaften und Rohstoffe (BGR), Hannover, Germany
Schmidt K, Paul SAL, Achterberg EP (2022) Assessing the availability of trace metals including rare earth elements in deep ocean waters of the Clarion Clipperton Zone, NE Pacific: Application of an in situ DGT passive sampling method. TrAC Trends Anal Chem 155:116657. https://doi.org/10.1016/j.trac.2022.116657
Schoening T, Purser A, Langenkämper D et al. (2020) Megafauna community assessment of polymetallic-nodule fields with cameras: platform and methodology comparison. Biogeosciences 17:3115–3133. https://doi.org/10.5194/bg-17-3115-2020
Sharma R (2000) Assessment of Impact on Seafloor Features in INDEX Area. Mar Georesources Geotechnol 18:237–250. https://doi.org/10.1080/10641190009353791
Sharma R, Rao AS (1992) Geological factors associated with megabenthic activity in the central Indian Basin. Deep Sea Res Part Oceanogr Res Pap 39:705–713. https://doi.org/10.1016/0198-0149(92)90096-C
Simon-Lledó E, Bett BJ, Huvenne VAI et al. (2019a) Megafaunal variation in the abyssal landscape of the Clarion Clipperton Zone. Prog Oceanogr 170:119–133. https://doi.org/10.1016/j.pocean.2018.11.003
Simon-Lledó E, Bett BJ, Huvenne VAI et al. (2019b) Ecology of a polymetallic nodule occurrence gradient: Implications for deep-sea mining. Limnol Oceanogr 64:1883–1894. https://doi.org/10.1002/lno.11157
Simon-Lledó E, Bett BJ, Huvenne VAI et al. (2019c) Biological effects 26 years after simulated deep-sea mining. Sci Rep 9. https://doi.org/10.1038/s41598-019-44492-w
Simon-Lledó E, Pomee C, Ahokava A et al. (2020) Multi-scale variations in invertebrate and fish megafauna in the mid-eastern Clarion Clipperton Zone. Prog Oceanogr 187:102405. https://doi.org/10.1016/j.pocean.2020.102405
Simon-Lledó E, Thompson S, Yool A et al. (2019d) Preliminary observations of the abyssal megafauna of Kiribati. Front Mar Sci 6:605. https://doi.org/10.3389/fmars.2019.00605
Smith CR, De Leo FC, Bernardino AF et al. (2008) Abyssal food limitation, ecosystem structure and climate change. Trends Ecol Evol 23:518–528. https://doi.org/10.1016/j.tree.2008.05.002
Stratmann T, Lins L, Purser A et al. (2018a) Abyssal plain faunal carbon flows remain depressed 26 years after a simulated deep-sea mining disturbance. Biogeosciences 15:4131–4145. https://doi.org/10.5194/bg-15-4131-2018
Stratmann T, Soetaert K, Kersken D, van Oevelen D (2021) Polymetallic nodules are essential for food-web integrity of a prospective deep-seabed mining area in Pacific abyssal plains. Sci Rep 11:12238. https://doi.org/10.1038/s41598-021-91703-4
Stratmann T, Voorsmit I, Gebruk A et al. (2018b) Recovery of Holothuroidea population density, community composition, and respiration activity after a deep-sea disturbance experiment. Limnol Oceanogr 63:2140–2153. https://doi.org/10.1002/lno.10929
Taboada S, Riesgo A, Wiklund H et al. (2018) Implications of population connectivity studies for the design of marine protected areas in the deep sea: An example of a demosponge from the Clarion-Clipperton Zone. Mol Ecol 27:4657–4679. https://doi.org/10.1111/mec.14888
Tavares DC, Moura JF, Acevedo-Trejos E, Merico A (2019) Traits shared by marine megafauna and their relationships with ecosystem functions and services. Front Mar Sci 6:262. https://doi.org/10.3389/fmars.2019.00262
Thiel H, Schriever G, Ahnert A et al. (2001) The large-scale environmental impact experiment DISCOL—reflection and foresight. Deep Sea Res Part II Top Stud Oceanogr 48:3869–3882. https://doi.org/10.1016/S0967-0645(01)00071-6
Thiel H, Schriever G, Bussau C, Borowski C (1993) Manganese nodule crevice fauna. Deep Sea Res Part Oceanogr Res Pap 40:419–423. https://doi.org/10.1016/0967-0637(93)90012-R
Tilot V, Ormond R, Moreno Navas J, Catalá TS (2018) The Benthic Megafaunal Assemblages of the CCZ (Eastern Pacific) and an Approach to their Management in the Face of Threatened Anthropogenic Impacts. Front Mar Sci 5:7. https://doi.org/10.3389/fmars.2018.00007
Uhlenkott K, Simon-Lledó E, Vink A, Martínez Arbizu P (submitted) Habitat heterogeneity enhances megafaunal biodiversity at bathymetric elevations in the Clarion Clipperton Fracture Zone
Uhlenkott K, Simon-Lledó E, Vink A, Martínez Arbizu P (2022) Investigating the benthic megafauna in the eastern Clarion Clipperton Fracture Zone (north-east Pacific) based on distribution models predicted with random forest. Sci Rep 12:8229. https://doi.org/10.1038/s41598-022-12323-0
Uhlenkott K, Vink A, Kuhn T, Martínez Arbizu P (2020) Predicting meiofauna abundance to define preservation and impact zones in a deep-sea mining context using random forest modelling. J Appl Ecol 57:1210–1221. https://doi.org/10.1111/1365-2664.13621
Vanreusel A, Hilario A, Ribeiro PA et al. (2016) Threatened by mining, polymetallic nodules are required to preserve abyssal epifauna. Sci Rep 6:26808. https://doi.org/10.1038/srep26808
Vink A, shipboard scientific party (in press) MANGAN 2021. Bundesanstalt für Geowissenschaften und Rohstoffe (BGR), Hannover, Germany
Volz JB, Mogollón JM, Geibert W et al. (2018) Natural spatial variability of depositional conditions, biogeochemical processes and element fluxes in sediments of the eastern Clarion-Clipperton Zone, Pacific Ocean. Deep Sea Res Part Oceanogr Res Pap 140:159–172. https://doi.org/10.1016/j.dsr.2018.08.006
Washburn TW, Jones DOB, Wei C-L, Smith CR (2021) Environmental Heterogeneity Throughout the Clarion-Clipperton Zone and the Potential Representativity of the APEI Network. Front Mar Sci 8:661685. https://doi.org/10.3389/fmars.2021.661685
Wedding LM, Friedlander AM, Kittinger JN et al. (2013) From principles to practice: a spatial approach to systematic conservation planning in the deep sea. Proc R Soc B Biol Sci 280:20131684. https://doi.org/10.1098/rspb.2013.1684
Acknowledgements
Many thanks are due to the anonymous reviewers for their constructive and helpful comments to improve the manuscript. We also want to thank captains, crew, and participants of RV Sonne, RV Kilo Moana, and MV Island Pride for their help and expertise during the expeditions into the BGR contract area. Furthermore, we want to thank a very large number of scientists and students who investigated the megafaunal community in the BGR contract area over the last 10 years. Thanks to the GRG who contributed to the acquisition, processing, and interpretation of the data presented.
Pictures and sample information presented in this study originate from the MANGAN exploration project for polymetallic nodules by the German Federal Institute for Geosciences and Natural Resources (BGR) on behalf of the Federal Ministry for Economic Affairs and Climate Action. Exploration activities are carried out in compliance with the regulations associated with exploration as issued by the International Seabed Authority.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research was partly funded through contractual work assigned by the BGR and partly by the German Federal Ministry of Education and Research (BMBF) (grant numbers 03F0812E and 03F0707E) as a contribution to the European project JPI-Oceans “Ecological Aspects of Deep-Sea Mining.”
Author information
Authors and Affiliations
Contributions
KM and KU drafted the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
Does not apply.
Sampling and field studies
Does not apply.
Additional information
Communicated by S. Kaiser
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Katja Uhlenkott and Klaas Meyn are co-first authors
This article is a contribution to the Topical Collection Biodiversity in Abyssal Polymetallic Nodule Areas
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Uhlenkott, K., Meyn, K., Vink, A. et al. A review of megafauna diversity and abundance in an exploration area for polymetallic nodules in the eastern part of the Clarion Clipperton Fracture Zone (North East Pacific), and implications for potential future deep-sea mining in this area. Mar. Biodivers. 53, 22 (2023). https://doi.org/10.1007/s12526-022-01326-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12526-022-01326-9