Abstract
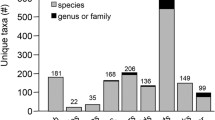
The global transhipment of ballast water and associated flora and fauna by cargo vessels has increased dramatically in recent decades. Invertebrate species are frequently carried in ballast water and sediment, although identification of diapausing eggs can be extremely problematic. Here we test the application of DNA barcoding using mitochondrial cytochrome c oxidase subunit I and 16S rDNA to identify species from diapausing eggs collected in ballast sediment of ships. The accuracy of DNA barcoding identification was tested by comparing results from the molecular markers against each other, and by comparing barcoding results to traditional morphological identification of individuals hatched from diapausing eggs. Further, we explored two public genetic databases to determine the broader applicability of DNA barcodes. Of 289 diapausing eggs surveyed, sufficient DNA for barcoding was obtained from 96 individuals (33%). Unsuccessful DNA extractions from 67% of eggs in our study were most likely due to degraded condition of eggs. Of 96 eggs with successful DNA extraction, 61 (64%) were identified to species level, while 36% were identified to possible family/order level. Species level identifications were always consistent between methodologies. DNA barcoding was suitable for a wide range of taxa, including Branchiopoda, Copepoda, Rotifera, Bryozoa and Ascidia. Branchiopoda and Copepoda were respectively the best and worst represented groups in genetic databases. Though genetic databases remain incomplete, DNA barcoding resolved nearly double the number of species identified by traditional taxonomy (19 vs. 10). Notorious invaders are well represented in existing databases, rendering these NIS detectable using molecular methods. DNA barcoding provides a rapid and accurate approach to identification of invertebrate diapausing eggs that otherwise would be very difficult to identify.



Similar content being viewed by others
References
Adamowicz SJ, Petrusek A, Colbourne JK, Hebert PDN, Witt JDS (2009) The scale of divergence: a phylogenetic appraisal of intercontinental allopatric speciation in a passively dispersed freshwater zooplankton genus. Mol Phylogenet Evol 50:423–436
Bailey SA, Duggan IC, van Overdijk CDA, Jenkins PT, MacIsaac HJ (2003) Viability of invertebrate diapausing stages collected from residual ballast sediment of transoceanic vessels. Limnol Oceanogr 48:1701–1710
Bailey SA, Duggan IC, Jenkins PT, MacIsaac HJ (2005) Invertebrate resting stages in residual ballast sediment of transoceanic ships. Can J Fish Aquat Sci 62:1090–1103
Barrett RDH, Hebert PDN (2005) Identifying spiders through DNA barcodes. Can J Zool 83:481–491
Bax N, Carlton JT, Mathews-Amos A, Haedrich RL, Howarth FG, Purcell JE, Rieser A, Gray A (2001) The control of biological invasions in the world’s oceans. Conserv Biol 15:1234–1246
Bickford D, Lohman DJ, Sodhi NS, Ng PKL, Meier R, Winker K, Ingram KK, Das I (2007) Cryptic species as a window on diversity and conservation. Trends Ecol Evol 22:148–155
Briski E, Van Stappen G, Bossier P, Sorgeloos P (2008) Laboratory production of early hatching Artemia sp. cysts by selection. Aquaculture 282:19–25
Briski E, Bailey SA, Cristescu ME, MacIsaac HJ (2010) Efficacy of ‘saltwater flushing’ in protecting the Great Lakes from biological invasions by invertebrate eggs in ships’ ballast sediment. Freshwater Biol 55(11):2414–2424. doi:10.1111/j.1365-2427.2010.02449.x
Brown B, Emberson RM, Paterson AM (1999) Mitochondrial COI and II provide useful markers for Weiseana (Lepidoptera, Hepialidae) species identification. Bull Entomol Res 89:287–294
Bucklin A, Guarnieri M, Hill RS, Bentley AM, Kaartvedt S (1999) Taxonomic and systematic assessment of planktonic copepods using mitochondrial COI sequence variation and competitive, species-specific PCR. Hydrobiologia 401:239–254
Bucklin A, Frost BW, Bradford-Grieve J, Allen LD, Copley NJ (2003) Molecular systematic and phylogenetic assessment of 34 calanoid copepod species of the Calanidae and Clausocalanidae. Mar Biol 142:333–343
Byers JE, Reichard S, Randall JM, Parker IM, Smith CS, Lonsdale WM, Atkinson IAE, Seasted TR, Williamson M, Chornesky E, Hayes D (2002) Directing research to reduce the impacts of nonindigenous species. Conserv Biol 16:630–640
Carlton JT (2009) Deep invasion ecology and the assembly of communities in historical time. In: Rilov G, Crooks JA (eds) Biological invasions in marine ecosystems: ecological, management, and geografic perspectives. Springer, Berlin, pp 13–56
Carlton JT, Geller JB (1993) Ecological roulette: the global transport of nonindigenous marine organisms. Science 261:78–82
Carvalho GR, Wolf HG (1989) Resting eggs of lake-Daphnia I. Distribution, abundance and hatching of eggs collected from various depths in lake sediment. Freshw Biol 22:459–470
Colautti RI, Grigorovich IA, MacIsaac HJ (2006) Propagule pressure: a null model for biological invasions. Biol Invasions 8:1023–1037
Costa FO, de Waard JR, Boutillier J, Ratnasingham S, Dooh RT, Hajibabaei M, Hebert PDN (2007) Biological identifications through DNA barcodes: the case of the Crustacea. Can J Fish Aquat Sci 64:272–295
Dahms HU (1995) Dormancy in the copepoda––an overview. Hydrobiologia 306:199–211
Darling JA, Blum MJ (2007) DNA-based methods for monitoring invasive species: a review and prospectus. Biol Invasions 9:751–765
Duggan IC, van Overdijk CDA, Bailey SA, Jenkins PT, Limen H, MacIsaac HJ (2005) Invertebrates associated with residual ballast water and sediments of cargo-carrying ships entering the Great Lakes. Can J Fish Aquat Sci 62:2463–2474
Duggan IC, Bailey SA, van Overdijk CDA, MacIsaac HJ (2006) Invasion risk of active and diapausing invertebrates from residual ballast in ships entering Chesapeake Bay. Mar Ecol Prog Ser 324:57–66
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Forró L, Korovchinsky NM, Kotov AA, Petrusek A (2008) Global diversity of cladocerans (Cladocera; Crustacea) in freshwater. Hydrobiologia 595:177–184
Geller JB, Darling JA, Carlton JT (2010) Genetic perspectives on marine biological invasions. Annu Rev Mar Sci 2:367–393
Giangrande A (2003) Biodiversity, conservation, and the ‘Taxonomic impediment’. Aquatic Conserv: Mar Freshw Ecosyst 13:451–459
Gilbert JJ (2004) Females from resting eggs and parthenogenetic eggs in the rotifer Brachionus calyciflorus: lipid droplets, starvation resistance and reproduction. Freshw Biol 49:1505–1515
Gómez A, Carvalho GR, Lunt DH (2000) Phylogeography and regional endemism of a passively dispersing zooplankter: mitochondrial DNA variation in rotifer resting egg banks. Proc R Soc Lond B Biol Sci 267:2189–2197
Gómez A, Hughes RN, Wright PJ, Carvalho GR, Lunt DH (2007) Mitochondrial DNA phylogeography and mating compatibility reveal marked genetic structuring and speciation in the NE Atlantic bryozoans Celleporella hyalina. Mol Ecol 16:2173–2188
Grice GD, Marcus NH (1981) Dormant eggs of marine copepods. Oceanogr Mar Biol Ann Rev 19:125–140
Hairston NG (1996) Zooplankton egg banks as biotic reservoirs in changing environments. Limnol Oceanogr 41:1087–1092
Hajibabaei M, Singer GCA, Clare EL, Hebert PDN (2007) Design and applicability of DNA arrays and DNA barcodes in biodiversity monitoring. BMC Biol 5:24
Hayes KR, Barry SC (2008) Are there any consistent predictors of invasion success? Biol Invasions 10:483–506
Hebert PDN, Crease TJ (1980) Clonal co-existence in Daphnia pulex (Leydig): another planktonic paradox. Science 207:1363–1365
Hebert PDN, Remigio EA, Colbourne JK, Taylor DJ, Wilson CC (2002) Accelerated molecular evolution in halophilic crustaceans. Evolution 56:909–926
Hebert PDN, Cywinska A, Ball SL, deWaard JR (2003) Biological identifications through DNA barcodes. Proc R Soc Lond B Biol Sci 270:313–321
Hebert PDN, Stoeckle MY, Zemlak TS, Francis CM (2004) Identification of birds through DNA barcodes. PLoS Biol 2:1657–1663
Imaizumi K, Akutsu T, Miyasaka S, Yoshino M (2007) Development of species identification tests targeting the 16S ribosomal RNA coding region in mitochondrial DNA. Int J Legal Med 121:184–191
Ivanova NV, Zemlak TS, Hanner RH, Hebert PDN (2007) Universal primer cocktails for fish DNA barcoding. Mol Ecol Notes 7:544–548
Janzen DH, Hajibabaei M, Burns JM, Hallwachs W, Remigio E, Hebert PDN (2005) Wedding biodiversity inventory of a large complex Lepidoptera fauna with DNA barcoding. Philos Trans R Soc Lond B Biol Sci 360:1835–1845
Jarman SN, Elliott NG (2000) DNA evidence for morphological and cryptic Cenozoic speciations in the Anaspididae, ‘living fossils’ from the Triassic. J Evol Biol 13:624–633
Kiesling TL, Wilkinson E, Rabalais J, Ortner PB, McCabe MM, Fell JW (2002) Rapid identification of adult and naupliar stages of copepods using DNA hybridization methodology. Mar Biotechnol 4:30–39
Knowlton N (1993) Sibling species in the sea. Annu Rev Ecol Syst 24:189–216
Lavens P, Sorgeloos P (1996) Manual on the production and use of live food for aquaculture. FAO Fish Tech Pap Rome pp 1–8
Leppäkoski E, Gollasch S, Gruszka P, Ojaveer H, Olenin S, Panov V (2002) The Baltic––a sea of invaders. Can J Fish Aquat Sci 59:1175–1188
Lockwood JL, Cassey P, Blackburn TM (2009) The more you introduce the more you get: the role of colonization pressure and propagule pressure in invasion ecology. Divers Distrib 15:904–910
Lodge DM, Williams S, MacIsaac HJ, Hayes KR, Leung B, Reichard S, Mack RN, Moyle PB, Smith M, Andow DA, Carlton JT, McMichael A (2006) Biological invasions: recommendations for US policy and management. Ecol Appl 16:2035–2054
Lopez JV, Culver M, Stephens JC, Johnson WE, O’Brien SJ (1997) Rates of nuclear and cytoplasmic mitochondrial DNA sequence divergence in mammals. Mol Biol Evol 14:277–286
Meyer CP, Paulay G (2005) DNA barcoding: error rates based on comprehensive sampling. PLoS Biol 3:e422
Monaghan MT, Balke M, Gregory TR, Volger AP (2005) DNA-based species delineation in tropical beetles using mitochondrial and nuclear markers. Philos Trans R Soc Lond B Biol Sci 360:1925–1933
Montero-Pau J, Gómez A, Muñoz J (2008) Application of an inexpensive and high-throughput genomic DNA extraction method for the molecular ecology of zooplanktonic diapausing eggs. Limnol Oceanogr Methods 6:218–222
Palumbi S (1996) Nucleic acids II: the polymerase chain reaction. In: Hillis D, Mable B, Moritz C (eds) Molecular systematics. Sinauer, Sunderland, pp 205–247
Pauwels K, Stoks R, Verbiest A, De Meester L (2007) Biochemical adaptation for dormancy in subitaneous and dormant eggs of Daphnia magna. Hydrobiologia 594:91–96
Puillandre N, Strong EE, Bouchet P, Boisselier M-C, Couloux A, Samadi S (2009) Identifying gastropod spawn from DNA barcodes: possible but not yet practible. Mol Ecol Resour 9:1311–1321
Ricciardi A (2006) Patterns of invasion in the Laurentian Great Lakes in relation to changes in vector activity. Divers Distrib 12:425–433
Rombouts I, Beaugrand G, Ibaňez F, Gasparini S, Chiba S, Legendre L (2009) Global latitudinal variations in marine copepod diversity and environmental factors. Proc R Soc B 276(1670):3053–3062
Rugman-Jones PF, Hoddle MS, Mound LA, Stouthamer R (2006) Molecular identification key for pest species of Scirtothrips (Thysanoptera: Thripidaae). J Econ Entomol 99:1813–1819
Ruiz GM, Fofonoff PW, Carlton JT, Wonham MJ, Hines AH (2000) Invasion of coastal marine communities in North America: apparent patterns, processes and biases. Annu Rev Ecol Syst 31:481–531
Ruppert EE, Fox RS, Barnes RD (2004) Invertebrate zoology. Thomson Learning, USA
Schubart CD, Santl T, Koller P (2008) Mitochondrial patterns of intra- and interspecific differentiation among endemic freshwater crabs of ancient lakes in Sulawesi. Contrib Zool 77:83–90
Schwartz SS, Hebert PDN (1987) Methods for the activation of the resting eggs of Daphnia. Freshw Biol 17:373–379
Shouche JS, Patole MS (2000) Sequence analysis of mitochondrial 16S ribosomal RNA gene fragment from seven mosquito species. J Biosci 25:361–366
Simberloff D (2009) We can eliminate invasions or live with them. Successful management projects. Biol Invasions 11:149–157
Simberloff D, Parker IM, Windle PN (2005) Introduced species policy, management, and future research needs. Front Ecol Environ 3:12–20
Sylvester F, MacIsaac HJ (2010) Is vessel hull fouling an invasion threat to the Great Lakes? Divers Distrib 16:132–143
Ward RD, Zemlak TS, Innes BH, Last PR, Hebert PDN (2005) DNA barcoding Australia’s fish species. Phil Trans R Soc Land B Biol Sci 360:1847–1857
Wong EH-K, Hanner RH (2008) DNA barcoding detects market substitution in North American seafood. Food Res Int 41:828–837
Wonham MJ, Carlton JT (2005) Trends in marine biological invasions at local and regional scales: the Northeast Pacific Ocean as a model system. Biol Invasions 7:369–392
Zhang D-X, Hewitt GM (1997) Assessment of the universality and utility of a set of conserved mitochondrial COI primers in insects. Insect Mol Biol 6:143–150
Acknowledgments
We thank the Shipping Federation of Canada and the multiple shipping companies that facilitated access to vessels, and our ballast sampling teams: C. van Overdijk, A.M. Weise, O. Casas-Monroy, N. Simard, J.-Y. Couture, M. Huot and Dr. C. McKindsey. We are grateful for taxonomic assistance from Drs. S.I. Dodson, J.R. Cordell and P. Hudson, and laboratory support from R. Tedla, S. Ross and H. Coker. Great thanks to Drs. Aibin Zhan and Francisco Sylvester for commenting on an early version of the manuscript. Comments from two anonymous reviewers are gratefully acknowledged. This research was supported by NSERC’s Canadian Aquatic Invasive Species Network, Transport Canada, Fisheries and Oceans Canada, by NSERC Discovery Grants to MEC, SAB and HJM, and by a DFO Invasive Species Research Chair to HJM.
Author information
Authors and Affiliations
Corresponding author
Appendix
Rights and permissions
About this article
Cite this article
Briski, E., Cristescu, M.E., Bailey, S.A. et al. Use of DNA barcoding to detect invertebrate invasive species from diapausing eggs. Biol Invasions 13, 1325–1340 (2011). https://doi.org/10.1007/s10530-010-9892-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-010-9892-7