Abstract
In Europe, turbot aquaculture has a high potential for sustainable production, but the low tolerance to fishmeal replacement in the diet represents a big issue. Therefore, this study investigated the effects of more sustainable feed formulations on growth and feed performance, as well as nutritional status of juvenile turbot in recirculating aquaculture systems. In a 16-week feeding trial with 20 g juvenile turbot, one control diet containing traditional fishmeal, fish oil and soy products and two experimental diets where 20% of the fishmeal was replaced either with processed animal proteins (PAP) or with terrestrial plant proteins (PLANT) were tested. Irrespective of diets, growth performance was similar between groups, whereas the feed performance was significantly reduced in fish of the PAP group compared to the control. Comparing growth, feed utilisation and biochemical parameters, the results indicate that the fish fed on PAP diet had the lowest performance. Fish fed the PLANT diet had similar feed utilisation compared to the control, whereas parameters of the nutritional status, such as condition factor, hepato-somatic index and glycogen content showed reduced levels after 16 weeks. These effects in biochemical parameters are within the physiological range and therefore not the cause of negative performance. Since growth was unaffected, the lower feed performance of fish that were fed the PAP formulation might be balanced by the cost efficient formulation in comparison to the commercial and the PLANT formulations. Present study highlights the suitability of alternative food formulation for farmed fish.
Similar content being viewed by others
Introduction
Aquaculture has the potential to ensure a reliable supply of seafood for the globally increasing demands and sustainable growth. In order to conserve and sustainably use aquatic resources, the reduction of the environmental footprint of aquaculture practices has become a high priority for the scientific community, producers and consumers. One of the major concerns is the challenge to feed farmed fish with diets that are nutritious but at the same time economically and environmentally sustainable (Glencross et al. 2020). In the last decades, research efforts focused on the identification of major nutritional requirements for important farmed fish such as trout, salmon, sea bass or seabream (FAO 2020; Naylor et al. 2021). These efforts set the foundation for substitution of fishmeal and fish oil originating from wild pelagic fish with other sources (Hardy and Barrows 2003). This resulted in diet formulations with reduced fish content that improve growth and feed performance (Olsen and Hasan 2012).
However, in many carnivorous farmed fish, a total replacement of fish products in the diets is still not feasible. In order to reduce dependence on traditional fishmeal and fish oil, the use of fishery and aquaculture by-products is a good alternative for sustainable aquafeeds (Bendiksen et al. 2011; Forster et al. 2005; Hua et al. 2019; Whiteman and Gatlin 2005). Processed raw materials such as hydrolysates are more energy efficient than fishmeal from by-products and were shown to improve growth and feed performance in farmed fish (Siddik et al. 2020).
Terrestrial plant materials are commonly integrated into commercial fish diets (Abdel-Latif et al. 2022; Gatlin et al. 2007; Naylor et al. 2021; Tacon et al. 2011) enabling even fish-free diet formulations for carnivorous fish. In the case of soybean, however, these products are often associated with unsustainable production, long transportation and a high proportion of genetically modified strains. Furthermore, soybean meals and other vegetable ingredients introduce anti-nutrients, giving rise to a number of problems for the fish such as enteritis and reduced nutrient uptake and bioavailability (Baeverfjord and Krogdahl 1996; Kaushik et al. 1995; Storebakken et al. 1998). This can be offset—at least to a certain point—by refining the plant protein sources (Glencross 2016; Jia et al. 2022; Naylor et al. 2009; Refstie et al. 2005), which, however, introduces costs and results in a trade-off between fish welfare/health and feed cost. Other alternative protein and oil crops such as pea, rapeseed and lupines proved to be suitable for fish feeds (Burel et al. 2000b; Glencross et al. 2011; Omnes et al. 2015; Øverland et al. 2009; Zhang et al. 2012). However, the availability at a competitive price and regular supply in sufficient quality is still a major issue that needs to be solved (Bähr et al. 2014; Glencross et al. 2020). Moreover, many consumers question whether plant materials are an acceptable and appropriate feed ingredient for carnivorous fish (Feucht and Zander 2015).
Plant material as a basic commodity is used in a wide range of human consumption, feed for terrestrial livestock, biofuels and many other industrial applications. Therefore, the competition is high, and aquaculture feed producers should avoid to totally rely on plant materials. Therefore, researchers and feed producers emphasise that a broader range of alternatives is needed to facilitate the predicted increase in fed aquaculture production (FAO 2020; Matos et al. 2017). Since the European crisis of the mad cow disease in 1990, terrestrial animal proteins were mostly banned from farmed animal feed formulations. Therefore, research on PAPs in fish feeds is scarce until recently. However, recent studies show that PAPs are suitable alternatives to fishmeal in fish diets (Campos et al. 2017; Karapanagiotidis et al. 2019; Lu et al. 2015; Wang et al. 2015; Wu et al. 2018). However, in 2013 non-ruminant PAPs (processed animal proteins) were re-authorised in the EU under very specific regulations allowing correctly categorised PAP in aquafeeds. The availability in large amounts in the EU and elsewhere as a by-product from food production and its nutritional value qualifies PAPs as a sustainable feed ingredient for fish (Tacon et al. 2011).
Recently authorised as novel food and feed in the EU, insect derived products, such as protein and lipids, are valuable ingredients for aquaculture feeds. Insects can valorise unused plant material, not suitable for human consumption, and transform it into valuable nutrients (Newton et al. 2005; Van Huis 2013). They are also part of the natural diet of many freshwater and marine fish species (Henry et al. 2015). Meals derived from the black soldier fly (Hermetia illucens) or mealworm (Tenebrio molitor) were already successfully tested in fish diets for carnivorous fish species such as Atlantic salmon (Salmo salar) (Li et al. 2020), rainbow trout (Oncorhynchus mykiss) (Jozefiak et al. 2019; Rema et al. 2019; Stadtlander et al. 2017) and red seabream (Pagrus major) (Ido et al. 2019).
Other feed ingredients, such as micro- and macroalgae and microbial meals, are emerging as suitable protein and lipid sources for aquafeeds. Microbial biomass, which is produced as a by-product from food, beer and biogas production, can be a valuable ingredient in aquafeeds (Aas et al. 2006; Bendiksen et al. 2011; Oliva-Teles and Goncalves 2001; Olsen and Hasan 2012; San Martin et al. 2020; Tacon et al. 2011). In particular, microalgae are a valuable source with essential fatty acids in diets with a low level of fish oil. Additionally, algae and yeast can act as functional ingredients, increasing the health of farmed fish and crustaceans (Dineshbabu et al. 2019; Refstie et al. 2010; Vallejos-Vidal et al. 2016; Wan et al. 2019).
Novel feed formulations with a broad spectrum of ingredients can balance the ingredients’ quality, cost and availability, but most importantly, they need to satisfy the nutritional requirements of the farmed species. Thereby the effects of integrating alternative feed ingredients on fish performance and nutritional status have to be validated. In comparison to fishmeal, alternative ingredients differ in nutritional composition, digestibility of nutrients and availability of minerals (Glencross 2016; Sugiura et al. 1998). This may affect growth, nutrient utilisation and whole body composition of carnivorous fish and lead to an altered energy metabolism and energy allocation. Plant-based and carbohydrate-rich diets influenced the energy reserves, such as the hepatic content of glycogen and lipid in Atlantic salmon, rainbow trout (Krogdahl et al. 2004), Gilthead seabream (Sparus aurata) (Robaina et al. 1995) and turbot (Scophthalmus maximus) (Miao et al. 2016). Furthermore, plant-based diets affected the mineral composition and availability in rainbow trout (Antony Jesu Prabhu et al. 2018; Read et al. 2014) and Atlantic salmon (Silva et al. 2019; Storebakken et al. 2000).
Turbot is an important species in EU aquaculture due to its high value and reputation and low competition with fishery production (EUMOFA 2018). It has a high potential for sustainable production due to the controlled farming cycle, production practices (RAS and flow-through systems) and its robustness, enabling high-density farming and domestication (FAO, 2005, 2005; Aksungur et al. 2007; Bischoff et al. 2018; EUMOFA 2018; Li et al. 2013). However, as a carnivore, turbot has a low tolerance to fishmeal reduction (Burel et al. 2000a, 2000b; Nagel et al. 2012; von Danwitz et al. 2016) and is a sensitive and thus suitable candidate for testing novel feed formulations. Therefore, the present study aims to evaluate the effects of two novel feed formulations for sustainable turbot production, with moderate fishmeal replacement and using feed ingredients of terrestrial animal and plant origin, on the growth and feed performance, apparent digestibility of nutrients, energy storage and apparent availability of minerals and trace elements.
Material and methods
Experimental diets
All experimental diets were formulated to be isonitrogenous (530 g kg−1). Due to species’ behaviour and size, 3 mm pellets with positive buoyancy (floating) were manufactured by extrusion at SPAROS LDA (Olhão, Portugal). All diets, including the control diet, were produced using the same facility and extrusion parameters to minimise technological differences. There were three treatments, including two novel formulations and one control diet, which was mimicking a typical current commercial formulation used for turbot. In the control diet, the main protein sources were fishmeal (500 g kg−1), wheat gluten (110 g kg−1) and soy protein concentrate (100 g kg−1). In the two experimental diets, the commercial fishmeal was fully replaced with fish by-products (meal and hydrolysates), and the overall fish-derived content was reduced by 20% to 400 g kg−1. The remaining protein was sourced with emerging ingredients such as insect meal, single cell meal and algae meal. Soy-derived ingredients were replaced by pea protein and pea starch. Furthermore, in all experimental diets, DHA-rich algae and rapeseed oil replaced 60% of fish oil. The content of the respective experimental diets as well as the control diet is shown in Tables 1 and 2. Once the experimental feeds were produced, they were delivered from Portugal to the experimental facility at the Alfred Wegener Institute Helmholtz Centre for Polar and Marine Research (AWI) in Bremerhaven (Germany). Before and during the trials, the feed was stored at 4 °C to ensure continuous quality of the diets throughout the feeding experiment.
Experimental setup
Juvenile turbot (Scophthalmus maximus) were purchased from France Turbot (L'Épine, France), transferred in specified transport containers overland to the recirculating aquaculture systems (RAS) of the Centre for Aquaculture Research (ZAF) at AWI, acclimated to the RAS for 2 weeks prior to starting the 16 week (112 days) experimental trial. A total of 750 turbots with a mean weight (± SD) of 20.2 ± 0.4 g and a mean total length of 10.1 ± 0.1 cm were randomly distributed into 15 tanks (50 fish per tank, 5 tanks per diet). The RAS consisted of 36 tanks, each with a bottom area of 1 m2 and a volume of approx. 700 L. The condition of the process water was monitored constantly with a SC 1000 Multiparameter Universal Controller (Hach Lange GmbH, Germany), and the nutrient concentration was measured with the QuAAtro39 AutoAnalyzer (SEAL Analytical, Germany) twice a week (see Table 3).
The fish were fed twice a day (9 am and 2 pm) ad libitum. After the fish were fed in the afternoon (30 min later), the remaining pellets were netted (mesh size 1 mm) from the tanks, dried for 24 h at 50 °C and weighed. To account for potential weight loss of the non-eaten pellets, duplicates of each experimental diet (2 g each) were incubated at 16 °C and 100 cycles per minute in 100 mL water which was taken from the experimental recirculation system (30 ‰ salinity) (Obaldo et al. 2002). After 30 min, the content was sieved (mesh size 1 mm), the collected pellets were dried for 24 h at 50 °C and weighted. The weight loss was used to calculate the loss factor for later correction of the recovered non-eaten pellets (see Formula (6)).
Measurements and sampling
Fish were weighed to 0.2 g precision and measured in length to 0.5 cm precision every 4 weeks. At the end of the 16-week trial, 6 individuals from each of the 15 tanks were sampled, from which 3 fish were used for tissue sampling to determine the energy reserves and 3 fish per tank were sampled as whole fish for proximate and mineral analysis. Fish were anaesthetized with 500 mg L−1 tricaine methanesulfonate (MS-222; Sigma Aldrich, Germany). After recording weight (precision 0.01 g) and length (precision 0.5 cm), fish were sacrificed and tissues (liver and fillet without skin) were rapidly sampled on ice. The liver of three fish per tank (n = 15 fish per diet) was weighted with 0.0001 g precision to determine the hepato-somatic index (HSI). Both tissues were frozen in liquid nitrogen and stored at − 80 °C until further analysis. For digestibility analysis, the faeces were sampled by stripping anaesthetized fish and pooled from one tank, centrifuged at 4 °C and 3,000 × g for 5 min, and the pellets were frozen at − 80 °C until further analysis. To gain sufficient tissue mass, the whole fish bodies were pooled (from the 3 fish taken per tank), cut into small pieces and stored at − 20 °C until further analysis.
Chemical analysis of diets, whole body and faeces
The chemical analysis of the diets was conducted in duplicates (see Table 2) and of the whole body and faeces as pooled replicates per tank (n = 5 tanks per diet). The whole body samples were minced frozen using a meat grinder (MADO Primus, Germany), refrozen at − 20 °C and then freeze-dried for 48 h. The samples of the experimental diets and faeces were freeze-dried for 24 h. The experimental diets and whole body samples were further homogenised in a knife grinder (5000 rpm, 30 s, Grindomix GM 200, Retsch, Germany).
The moisture content, ash, crude protein, crude lipid and energy of the experimental diets, whole body fish and faeces was determined after AOAC (1980). Moisture content of the feeds was determined by drying the samples at 105 °C for 24 h. The moisture content of the whole body and faeces was determined by freeze-drying. Total ash content was determined by combustion of the samples in a muffle oven at 550 °C for 6 h. The total nitrogen in the feed and whole body samples was determined following the automated Kjeldahl Method. Due to small sample volume in the faeces samples, the total nitrogen was determined after the Dumas method. For all samples, the measured total nitrogen was converted to equivalent crude protein (%) by the numerical factor of 6.25. Crude lipid was determined by acid hydrolysis. Gross energy was measured in an adiabatic bomb calorimeter (Model 6100; Parr Instrument, Germany).
For the analysis of the mineral content, 0.2 g of freeze-dried and homogenised samples of the experimental diets, whole body and faeces was digested in 3 mL nitric acid (HNO3) (65%, trace grade) in a microwave oven (CEM MARS5, Germany) according to DIN EN 13,805 (2014). After digestion, the samples were diluted with Milli-Q water to 50 mL. Calcium, potassium, magnesium, phosphorus, arsenic, copper, iron, manganese, yttrium and zinc concentrations were analysed in an ICP-OES (iCAP7400; Fisher Scientific, Germany). As reference fish muscle (ERM – BB422, EU) was used.
Glycogen and crude lipid content of liver and muscle tissue
Following the procedure described by Keppler and Decker (1988), glycogen content was determined photometrically after enzymatic hydrolysis of glycogen to glucose. Briefly, fillet and liver samples (3 individual fish per tank; 15 fish per diet in total) were grinded under liquid nitrogen, and approx. 200 mg tissue was homogenised in 5 × volume of ice-cold 0.6 M perchloric acid (PCA) (w:v). After one cycle of 20 s at 6000 rpm and 3 °C using Precellys 24 (Bertin Technologies, France), samples were sonicated for 2 min at 0 °C and 360 W (Branson Ultrasonics Sonifier 450; Fisher Scientific, Germany), and homogenates were immediately divided for the analysis of total and free glucose concentrations. Due to small volume, the individual samples of liver and muscle were pooled (n = 5 tanks per diet) for the crude lipid content. Following the method of Folch et al. (1957) and Postel et al. (2000), the lipids in the muscle and liver tissue were extracted with 2:1 dichloromethane-methanol (v/v) and an aqueous solution of 0.88% KCl (w:v). Crude lipid content was determined gravimetrically to the nearest 0.001 g and calculated as the percentage of lipids of tissue wet weight.
Data analysis (calculations and statistics)
The growth parameters were based on body weight (BW) and body length (BL) and calculated as follows.
The feed performance parameters, daily feed intake (DFI) and feed conversion ratio (FCR) were based on the feed intake (FI) in g of the offered amount of feed and the uneaten feed, which is corrected by the soluble loss factor. Total FI and WG for FCR were corrected for the lost biomass through mortalities and sampling.
The apparent digestibility (ADC) of the dietary nutrients and the apparent availability (AA) of minerals were based on the amount of the inert yttrium marker in the diet and faeces and the respective nutrient or element in faeces and diets.
The glycogen content was calculated based on the concentration of total glucose (ctotal glucose) subtracted by the concentration of free glucose (cfree glucose).
whereas ∆A = the change in absorption, Vassay total = the total measurement volume of the assay (ml), Ɛ = the coefficient of extinction at 339 nm (6.3 mL µmo−1 cm−1), d = the thickness of layer for the cuvette (1 cm), Vsample = the sample volume (mL), DF = the dilution factor and ctissue = the concentration of tissue wet weight in crude extract (mg mL−1).
The glucose concentration was converted to glycogen content using the molecular weight of the glucosyl moiety in glycogen with Mr = 162 g mol−1.
Statistical analysis
Statistical analysis was conducted with Sigma Plot (12.5, Systat Software, Germany). One-way analysis of variance (ANOVA) was used to determine significant differences between the treatments. Whenever there were statistically significant differences, an all pairwise multiple comparison procedure was performed using the Holm-Sidak method (overall significance level p = 0.050) to find the difference within the treatments. Values are given as means ± standard deviations.
Results
Growth and feed performance
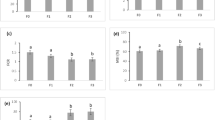
The experimental feed formulations did not significantly affect the growth of the juvenile turbots, and the survival during the experimental period was high with 0% mortalities (Table 4). After 16 weeks, the fish from the control group increased their weight 3.25 fold (65 g), whereas the fish fed with the experimental diets only increased their weight 3.10 fold (by an average of 62 g). The fish accepted all diets with a daily feed intake (DFI) of 0.99 ± 0.03% BW d−1 (n = 15 tanks). The feed conversion ratio (FCR) in the fish from the control group was significantly lower than in the fish from the PAP group, with no significant differences to the fish from the PLANT group. The protein efficiency ratio (PER) was significantly higher in the fish from the control group than in the PAP group with no significant differences to the fish from the PLANT group. The condition factor (CF) was significantly affected by the experimental diets (Table 4). The CFs of fish from PLANT group were significantly lower than of the fish in the control and the PAP group. However, the CF significantly increased in all treatments from the initial to the final (t-test; p = < 0.001).
Whole body composition, apparent digestibility and energy reserves
The whole body composition with moisture content, crude protein, ash content and energy content did not differ significantly between fish fed the different diets (Table 5). Fish from the PAP group had a significantly lower crude lipid content than the fish from the control group with no significant differences to the fish from the PLANT group. The apparent digestibility coefficients (ADC) of dry matter and crude protein were significantly higher in the fish from the control group compared to the fish from the experimental groups, whereas the ADC of energy was not affected (see Table 5).
The hepato-somatic index (HSI) of fish fed the control diet was significantly higher than that of the fish from the experimental groups (Table 6). The hepatic glycogen was significantly higher in the fish fed the control than in the fish fed the PLANT diet, with no significant difference to the fish from the PAP group. The hepatic lipid content, glycogen, and lipid content in the muscle of turbot showed no significant differences between groups (see Table 6).
Mineral analysis of the diets, mineral balance and apparent availability
The mineral content of the whole body showed no significant differences in the fish from all diets, except for arsenic and copper concentration in the PLANT-feeding fish that was significantly higher than in the control fish, with no significant differences to PAP (Table 7).
For all analysed minerals, the apparent availability (AA) was highest in the control diet compared to the experimental diets, except for potassium and sodium, where the AA in the control was lowest (Table 7). No significant differences were found in the availability of calcium, arsenic and zinc. The potassium and sodium availability in the control was significantly lower than in the experimental diets. In contrast, the availability of magnesium, copper and iron was in the control significantly higher than in the experimental diets. The phosphorus availability was in the control diets significantly higher than in the PLANT diet, with no significant differences to the PAP diet. The manganese availability was in the PLANT diet significantly lower than in the control and PAP diet.
Discussion
In the present study, the more sustainable feed formulations, in which fish-derived ingredients were reduced by 20%, resulted in juvenile turbot with similar growth comparing to the control (commercial-type feed) group. This is congruent with literature where decreased growth and feed performance were observed when more than 30–35% of fishmeal was replaced by processed animal protein (Dong et al. 2016), insect meal (Kroeckel et al. 2012) and plant protein (Bian et al. 2017; Bonaldo et al. 2015; Burel et al. 2000a; Fournier et al. 2004; Hermann et al. 2016; von Danwitz et al. 2016). Nevertheless and not unexpected, compared to controls, fish that were fed the slightly leaner experimental diets were less capable of building up energy reserves as the hepato-somatic index (HSI) and the slightly lower liver glycogen show, possibly augmented by a slightly lower apparent digestibility of dietary protein (2%) and energy (3%) of the control diet compared to the experimental diets. The fish in the PAP group had the poorest feed conversion, whereas other parameters showed no clear picture. In any case, the results of present study indicate that successful substitution of traditional fishmeal and fish oil can be achieved in turbot. It can further be speculated that an equal fraction of digestible energy contents between the different diets would have led to an even better similarity in fish performance between the diets of the GAIN project alternative formulations as it was shown with similar formulation in Gilthead seabream (Aragao et al. 2020). Even though the crude lipid content in the PLANT diet was 2% higher than in the control and PAP diet, a possible effect of this on the turbot can be negligible. The crude protein level (52%) used in present study is sufficiently high for turbot to minimise possible effects of the differing crude lipid level as previously observed in juvenile turbot (Sevgili et al. 2014).
Growth and feed performance
The relative growth rate (RGR) was similar between all groups, on average 1.27 ± 0.08% d−1 (n = 15 tanks) indicating that the different diets did not affect turbots’ energy allocation with respect to growth performance. In the present study, the RGR was higher by 0.01 percentage points compared to the specific growth rate (SGR), which is widely used in literature (i.e. Arnason et al. 2009; Bonaldo et al. 2011; Burel et al. 1996; Nagel et al. 2012). However, it is incorrect in concept to express the SGR as a percentage increase in daily weight, and therefore, the RGR is used instead (Hardy and Barrows 2003). The presented RGR results were lower than those of similar sized turbot based on the majority of available literature data (Arnason et al. 2009; Burel et al. 1996; Fuchs et al. 2015; Imsland et al. 1996; Nagel et al. 2017) but moderate to high compared to turbot in commercial RAS (Baer et al. 2011).
The feed conversion ratio (FCR) and protein efficiency ratio (PER) were significantly better in the control than in the PAP group. However, the differences between the FCRs in all diets were small with a mean value of 0.90 ± 0.03 (n = 15), and the daily feed intake (DFI) was approximately 0.99% BW d−1 resulting in a RGR in the expected range (Burel et al. 1996). Furthermore, the turbot strain used in the present study might have a lower growth rate per se, as turbot exhibit counter gradient variation (Imsland et al. 2000). Strains from lower latitudes, such as France, show generally lower growth and feed efficiency compared to populations from higher latitudes, such as Norway and Iceland (Imsland et al. 2001).
In present study, the differences in FCR might be overestimated due to the higher moisture content in the experimental diets compared to the control diet. The same pattern as for the FCR was observed in the protein efficiency ratio, with the highest value for the control, followed by PLANT and PAP. In line with literature, in the present study, the apparent digestibility coefficient (ADC) of protein in turbot decreased (> 90% in the control group with 450 g kg−1 fishmeal) when the fishmeal inclusion level is reduced (Bai et al. 2019; Bonaldo et al. 2011; Li et al. 2019; Liu et al. 2014b; Regost et al. 1999). When combining all feed performance indicators, the fish from the control group had the best performance followed by the PLANT group and the PAP group where fish showed the lowest performance.
Even though the condition factor (CF) of turbot from the PLANT group showed statistically a difference to the control and PAP group (2.07 vs. 2.11 and 2.10, respectively), the physiological relevance is minor and does not indicate poorer nutritional status. CFs above 2 indicate an overall good nutritional status of the fish, as presented CFs are similar to values of in previous studies (Fuchs et al. 2015; Nagel et al. 2017; von Danwitz et al. 2016; Wanka et al. 2019; Weiß and Buck 2017). In previous studies, a reduced CF was observed in turbot fed with plant-based diets (Bonaldo et al. 2015) and insect meal-based diets (Kroeckel et al. 2012) at a substitution/replacement level of more than 55%. In line with present study, reduced CF in fish fed with different PAPs was not observed in previous studies for European sea bass (Campos et al. 2017), Gilthead seabream (Karapanagiotidis et al. 2019) and rainbow trout (Lu et al. 2015). However, this might be biased by a lack of studies on this feed ingredient.
Nutritional and energy status
In this study, fish from the control group had a significantly higher HSI than the fish from the two experimental groups (1.8 vs. 1.5 and 1.5, respectively). The HSI of the control group is good for juvenile turbot (Bonaldo et al. 2015; Dietz et al. 2012; Nagel et al. 2017). The hepatic glycogen of the control, PAP and PLANT fed turbot (63.7 mg g−1, 48.0 mg g−1 vs. 46.4 mg g−1, respectively) followed a similar pattern indicating a positive correlation between glycogen as energy reserve in a good nutritional status and the HSI (Guerreiro et al. 2015a; Liu et al. 2014a; Miao et al. 2016; Zeng et al. 2015). Hepatic glycogen serves in many fish species as an energy reserve, and high glycogen deposition leads to increased liver weight in many fish species (Hemre et al. 2002). The liver lipid content was not affected by the diet, which is in line to a study by Guerreiro et al. (2015b) on European seabass that were fed with plant protein compared to fish protein. The effects of the diet on the hepatic lipid content might be minor since turbot does not store excess dietary lipid in the liver or muscle (Leknes et al. 2012; Liu et al. 2014a; Regost et al. 2001). The muscle glycogen and lipid content were not affected by the diets, whereas the muscle glycogen (1.9 mg g−1, calculated for all animals, irrespective of diet group) was on the lower range of 1–12 mg g−1 compared to previous studies (Miao et al. 2016; Pichavant et al. 2002; Soengas et al. 1995).
Considering the lower HSI and hepatic glycogen of the PAP and PLANT fed turbot, we can conclude that the experimental diets used in present study did alter the nutritional status of turbot to a certain degree without negatively affecting the growth. The decreased apparent digestibility of the experimental diets might have caused a reduced surplus on energy resulting in slightly smaller liver masses and thus HSI in PAP and PLANT fed turbot. Interestingly, it has been shown that the reduction and replacement of fishmeal with alternative feed ingredients could lead to contradicting results. Decreasing fishmeal content may lead to a decreased HSI (Bai et al. 2019; Gu et al. 2017; Kroeckel et al. 2012; von Danwitz et al. 2016; Wanka et al. 2019), unchanged HSI (Bonaldo et al. 2015; Fuchs et al. 2015; Wang et al. 2016; Weiß and Buck 2017) or even increased HSI (Dietz et al. 2012; Fournier et al. 2004; Nagel et al. 2017). This aspect might be worth investigating in more detail to unravel the observed variation in HSI, hepatic glycogen and lipid dependent on alternative feed ingredients.
Mineral balance, utilisation and availability
The concentrations of calcium, potassium, sodium, phosphorus, iron and manganese were lower in the control diet than in the experimental diets. The type of fishmeal used can explain the elevated ash, calcium and phosphorus content in the experimental diets. Fishmeal from fish by-products has a higher ash content containing much calcium and phosphorus due to a higher content of bones compared to traditional fishmeal (Olsen and Hasan 2012). These differences, however, did not significantly affect the concentration of minerals and trace element in the whole body of turbot, which are similar to those of other species (see meta-analysis by Antony Jesu Prabhu et al. 2016). However, the manganese concentration was twice as high as the maximum described for different fish species (Antony Jesu Prabhu et al. 2016) and for turbot in RAS (van Bussel et al. 2014). This might be due to accumulation effects in the whole body, which was already described in turbot (Ma et al. 2015) and Atlantic salmon parr (Lorentzen et al. 1996).
Even though there were no diet-dependent effects on the concentrations of calcium, arsenic and zinc, the apparent availability magnesium, copper and iron were significantly higher in the fish from the control group than in the fish fed with the experimental diets. Furthermore, the apparent availability of phosphorus and manganese was significantly reduced in fish fed the plant-based diet compared to the fish fed the control. Potassium and sodium had a significantly reduced apparent availability in the fish fed the control compared to the fish fed the experimental diets. Substances such as phytate in plant-based feed ingredients are known to bind minerals and, thus, reduce the availability of phosphorus, iron and zinc in fish (Kumar et al. 2012). The inclusion of rapeseed in diets leads to a reduced availability of phosphorus, manganese, iron and zinc but increased copper availability in turbot (von Danwitz et al. 2016). The potassium and sodium availability was in general high and was significantly higher in fish fed the experimental diets than in the control group (93% and 94% in PAP and PLANT vs. 88% in control). In contrast, the potassium availability in rainbow trout was higher in the fishmeal-based diet than in the plant-based diet (Antony Jesu Prabhu et al. 2015, 2018).
Since the mineral concentrations in the whole body are similar in the fish from all experimental groups, it can be concluded that the mineral and trace element demand was sufficiently covered and that the elevated mineral concentration in the experimental diets was balanced by elevated excretion rates.
Prediction of feed and production costs
The present results of growth performance indicate that alternative feed formulations can be used in commercial aquaculture for juvenile turbot. Since feed costs are the largest cost factor in the production, small differences in the FCR can balance feed costs and could make more cost efficient formulations attractive. The animal-based formulation (PAP) presented in this study has a lower cost with a commercial margin than the commercial-like control formulation, whereas the plant protein formulation (PLANT) is more expensive (see Table 8). Taking this study’s FCRs into consideration, the feed costs to produce one ton of turbot is still lower with the PAP formulation than the control and the PLANT formulation. Feeding juvenile turbot with the PAP formulation could lead to a cost reduction of 10% compared to the control, whereas feeding the PLANT formulation would increase the costs by 12%.
Conclusion
The present study highlighted that fish by-products are a suitable replacement for commercial fishmeal and that protein sources derived from terrestrial plants or animals can replace 20% of the overall fish-derived ingredients without compromising growth performance and body composition of juvenile turbot. These findings are a promising start for further research to find the optimal replacement of marine ingredients, in order to ensure acceptable feed utilisation and deviations from nutritional status. Overall, the alternative diet formulations may produce leaner fish, which have the potential for muscle growth rather than adiposity, and the slightly lowered apparent digestibility of protein suggests that waste production within a commercial aquaculture system would not be much higher than with feeding the control diet. Furthermore, the feed formulation based on processed animal protein (PAP) seems to be an economical feasible alternative for juvenile turbot since the lower feed related production costs balance the slightly poorer feed conversion. Further studies on turbot in the grow-out phase will investigate how a higher fishmeal replacement will affect the performance.
Besides the effects of the alternative feed formulations on fish performance, the economic and environmental benefits of the diets, the consumers’ acceptance of the diet formulations need to be considered. Alternative feed ingredients, sourced through circular economy processes, could be more environmentally sustainable (Maiolo et al. 2020) but may also increase production costs. Hereby particularly, insect and algae production could be included in an integrated multi trophic aquaculture (IMTA) system, which reduces the environmental impact by recycling of nutrients (Barrington et al. 2009; Milhazes-Cunha and Otero 2017). Many consumers are concerned that feed ingredients, such as by-products from terrestrial animals, may not be safe (Glencross et al. 2020). Furthermore, they express the concern that the feed formulations with high levels of plant ingredients might not be species appropriate and impair the animal welfare of cultured fish (Feucht and Zander 2015). Therefore, in addition to the marketing of more sustainable aquaculture products in Europe, such socio-economic aspects need to be considered when developing new and innovative fish diets for commercial important fish species.
Data availability
Data will be available over the PANGAEA ® Data publisher.
Code availability
Not applicable.
References
Aas TS, Grisdale-Helland B, Terjesen BF, Helland SJ (2006) Improved growth and nutrient utilisation in Atlantic salmon (Salmo salar) fed diets containing a bacterial protein meal. Aquaculture 259:365–376. https://doi.org/10.1016/j.aquaculture.2006.05.032
Abdel-Latif HMR, Abdel-Daim MM, Shukry M, Nowosad J, Kucharczyk D (2022) Benefits and applications of Moringa oleifera as a plant protein source in Aquafeed: a review. Aquaculture 547:737369. https://doi.org/10.1016/j.aquaculture.2021.737369
Aksungur N, Aksungur M, Akbulut B, Kutlu İ (2007) Effects of stocking density on growth performance, survival and food conversion ratio of turbot (Psetta maxima) in the net cages on the southeastern coast of the Black Sea. Turk J Fish Aquat Sci 7:147–152
Antony Jesu Prabhu P, Kaushik SJ, Mariojouls C, Surget A, Fontagne-Dicharry S, Schrama JW, Geurden I (2015) Comparison of endogenous loss and maintenance need for minerals in rainbow trout (Oncorhynchus mykiss) fed fishmeal or plant ingredient-based diets. Fish Physiol Biochem 41:243–253. https://doi.org/10.1007/s10695-014-0020-y
Antony Jesu Prabhu P, Schrama JW, Fontagné-Dicharry S, Mariojouls C, Surget A, Bueno M, Geurden I, Kaushik SJ (2018) Evaluating dietary supply of microminerals as a premix in a complete plant ingredient-based diet to juvenile rainbow trout (Oncorhynchus mykiss). Aquac Nutr 24:539–547. https://doi.org/10.1111/anu.12586
Antony Jesu Prabhu P, Schrama JW, Kaushik SJ (2016) Mineral requirements of fish: a systematic review. Rev Aquac 8:172–219. https://doi.org/10.1111/raq.12090
AOAC. 1980. 7. Animal Feed in Horwitz W, ed. Official methods of analysis. Washington: Association of Official Analytical Chemists.
Aragao C, Cabano M, Colen R, Fuentes J, Dias J (2020) Alternative formulations for gilthead seabream diets: towards a more sustainable production. Aquac Nutr 26:444–455. https://doi.org/10.1111/anu.13007
Arnason T, Bjornsson B, Steinarsson A, Oddgeirsson M (2009) Effects of temperature and body weight on growth rate and feed conversion ratio in turbot (Scophthalmus maximus). Aquaculture 295:218–225. https://doi.org/10.1016/j.aquaculture.2009.07.004
Baer A, Schulz C, Traulsen I, Krieter J (2011) Analysing the growth of turbot (Psetta maxima) in a commercial recirculation system with the use of three different growth models. Aquacult Int 19:497–511. https://doi.org/10.1007/s10499-010-9365-0
Baeverfjord G, Krogdahl A (1996) Development and regression of soybean meal induced enteritis in Atlantic salmon, Salmo salar L., distal intestine: a comparison with the intestines of fasted fish. J Fish Dis 19:375–387. https://doi.org/10.1046/j.1365-2761.1996.d01-92.x
Bähr M, Fechner A, Hasenkopf K, Mittermaier S, Jahreis G (2014) Chemical composition of dehulled seeds of selected lupin cultivars in comparison to pea and soya bean. Lwt-Food Science and Technology 59:587–590. https://doi.org/10.1016/j.lwt.2014.05.026
Bai N, Gu M, Liu M, Jia Q, Pan S, Zhang Z (2019) Corn gluten meal induces enteritis and decreases intestinal immunity and antioxidant capacity in turbot (Scophthalmus maximus) at high supplementation levels. PLoS One 14.https://doi.org/10.1371/journal.pone.0213867
Barrington K, Chopin T, Robinson S (2009) Integrated multi-trophic aquaculture (IMTA) in marine temperate waters. Integrated mariculture: a global review. FAO Fisheries and Aquaculture Technical Paper 529:7–46
Bendiksen EA, Johnsen CA, Olsen HJ, Jobling M (2011) Sustainable aquafeeds: progress towards reduced reliance upon marine ingredients in diets for farmed Atlantic salmon (Salmo salar L.). Aquaculture 314:132–139. https://doi.org/10.1016/j.aquaculture.2011.01.040
Bian F, Zhou H, He G, Wang C, Peng H, Pu X, Jiang H, Wang X, Mai K (2017) Effects of replacing fishmeal with different cottonseed meals on growth, feed utilization, haematological indexes, intestinal and liver morphology of juvenile turbot (Scophthalmus maximus L.). Aquac Nutr 23:1429–1439. https://doi.org/10.1111/anu.12518
Bischoff AA, Lutz M, Buck BH (2018) Juvenile turbot (Scophthalmus maximus L., 1758) farmed in a modern low-water exchange RAS device: growth performance using different diets and its commercial implications. J Appl Aquac 30:15–28. https://doi.org/10.1080/10454438.2017.1412378
Bonaldo A, Di Marco P, Petochi T, Marino G, Parma L, Fontanillas R, Koppe W, Mongile F, Finoia MG, Gatta PP (2015) Feeding turbot juveniles Psetta maxima L. with increasing dietary plant protein levels affects growth performance and fish welfare. Aquaculture Nutrition 21:401–413. https://doi.org/10.1111/anu.12170
Bonaldo A, Parma L, Mandrioli L, Sirri R, Fontanillas R, Badiani A, Gatta PP (2011) Increasing dietary plant proteins affects growth performance and ammonia excretion but not digestibility and gut histology in turbot (Psetta maxima) juveniles. Aquaculture 318:101–108. https://doi.org/10.1016/j.aquaculture.2011.05.003
Burel C, Boujard T, Kaushik SJ, Boeuf G, Van der Geyten S, Mol KA, Kuhn ER, Quinsac A, Krouti M, Ribaillier D (2000a) Potential of plant-protein sources as fish meal substitutes in diets for turbot (Psetta maxima): growth, nutrient utilisation and thyroid status. Aquaculture 188:363–382. https://doi.org/10.1016/S0044-8486(00)00342-2
Burel C, Boujard T, Tulli F, Kaushik SJ (2000b) Digestibility of extruded peas, extruded lupin, and rapeseed meal in rainbow trout (Oncorhynchus mykiss) and turbot (Psetta maxima). Aquaculture 188:285–298. https://doi.org/10.1016/S0044-8486(00)00337-9
Burel C, Person-Le Ruyet J, Gaumet F, Le-Roux A, Severe A, Boeuf G (1996) Effects of temperature on growth and metabolism in juvenile turbot. J Fish Biol 49:678–692. https://doi.org/10.1006/jfbi.1996.0196
Campos I, Matos E, Marques A, Valente LMP (2017) Hydrolyzed feather meal as a partial fishmeal replacement in diets for European seabass (Dicentrarchus labrax) juveniles. Aquaculture 476:152–159. https://doi.org/10.1016/j.aquaculture.2017.04.024
Dietz C, Kroeckel S, Schulz C, Susenbeth A (2012) Energy requirement for maintenance and efficiency of energy utilization for growth in juvenile turbot (Psetta maxima, L.): The effect of strain and replacement of dietary fish meal by wheat gluten. Aquaculture 358:98–107. https://doi.org/10.1016/j.aquaculture.2012.06.028
DIN (2014) Lebensmittel – Bestimmung von Elementspuren – Druckaufschluss; Deutsche Fassung EN 13805:2014: Deutsches Institut für Normung e. V.
Dineshbabu G, Goswami G, Kumar R, Sinha A, Das D (2019) Microalgae-nutritious, sustainable aqua- and animal feed source. Journal of Functional Foods 62.https://doi.org/10.1016/j.jff.2019.103545
Dong C, He G, Mai KS, Zhou HH, Xu W (2016) Palatability of water-soluble extracts of protein sources and replacement of fishmeal by a selected mixture of protein sources for juvenile turbot (Scophthalmus maximus). Journal of Ocean University of China 15:561–567. https://doi.org/10.1007/s11802-016-2898-8
EUMOFA (2018) Turbot in the EU. European Commission, Directorate-General for Maritime Affairs and Fisheries, Brussels
FAO (2005) Psetta maxima. (14.05.2020)
FAO (2020) The State of World Fisheries and Aquaculture 2020. Sustainability in action. Rome, Italy.
Feucht Y, Zander K (2015) Of earth ponds, flow-through and closed recirculation systems — German consumers’ understanding of sustainable aquaculture and its communication. Aquaculture 438:151–158. https://doi.org/10.1016/j.aquaculture.2015.01.005
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Forster I, Babbitt JK, Smiley S (2005) Comparison of the nutritional quality of fish meals made from by-products of the Alaska fishing industry in diets for pacific threadfin (Polydactylus sexfilis). J World Aquaculture Soc 36:530–537. https://doi.org/10.1111/j.1749-7345.2005.tb00401.x
Fournier V, Huelvan C, Desbruyeres E (2004) Incorporation of a mixture of plant feedstuffs as substitute for fish meal in diets of juvenile turbot (Psetta maxima). Aquaculture 236:451–465. https://doi.org/10.1016/j.aquaculture.2004.01.035
Fuchs VI, Schmidt J, Slater MJ, Zentek J, Buck BH, Steinhagen D (2015) The effect of supplementation with polysaccharides, nucleotides, acidifiers and Bacillus strains in fish meal and soy bean based diets on growth performance in juvenile turbot (Scophthalmus maximus). Aquaculture 437:243–251. https://doi.org/10.1016/j.aquaculture.2014.12.007
Gatlin DM et al (2007) Expanding the utilization of sustainable plant products in aquafeeds: a review. Aquac Res 38:551–579. https://doi.org/10.1111/j.1365-2109.2007.01704.x
Glencross B (2016) Understanding the nutritional and biological constraints of ingredients to optimize their application in aquaculture feeds. In: Nates SF (ed) Aquafeed Formulation. Academic Press, San Diego, pp 33–73
Glencross B, Rutherford N, Hawkins W (2011) A comparison of the growth performance of rainbow trout (Oncorhynchus mykiss) when fed soybean, narrow-leaf or yellow lupin meals in extruded diets. Aquac Nutr 17:317–325. https://doi.org/10.1111/j.1365-2095.2010.00765.x
Glencross BD, Baily J, Berntssen MHG, Hardy R, MacKenzie S, Tocher DR (2020) Risk assessment of the use of alternative animal and plant raw material resources in aquaculture feeds. Rev Aquac 12:703–758. https://doi.org/10.1111/raq.12347
Gu M, Bai N, Kortner TM (2017) Taurocholate supplementation attenuates the changes in growth performance, feed utilization, lipid digestion, liver abnormality and sterol metabolism in turbot (Scophthalmus maximus) fed high level of plant protein. Aquaculture 468:597–604. https://doi.org/10.1016/j.aquaculture.2016.11.022
Guerreiro I, Enes P, Merrifield D, Davies S, Oliva-Teles A (2015a) Effects of short-chain fructooligosaccharides on growth performance and hepatic intermediary metabolism in turbot (Scophthalmus maximus) reared at winter and summer temperatures. Aquac Nutr 21:433–443. https://doi.org/10.1111/anu.12175
Guerreiro I, Oliva-Teles A, Enes P (2015b) Improved glucose and lipid metabolism in European sea bass (Dicentrarchus labrax) fed short-chain fructooligosaccharides and xylooligosaccharides. Aquaculture 441:57–63. https://doi.org/10.1016/j.aquaculture.2015.02.015
Hardy RW, Barrows FT (2003) Diet formulation and manufacture. In: Halver JE, Hardy RW (eds) Fish Nutrition. Academic Press, San Diego, pp 505–600
Hemre GI, Mommsen TP, Krogdahl A (2002) Carbohydrates in fish nutrition: effects on growth, glucose metabolism and hepatic enzymes. Aquac Nutr 8:175–194. https://doi.org/10.1046/j.1365-2095.2002.00200.x
Henry M, Gasco L, Piccolo G, Fountoulaki E (2015) Review on the use of insects in the diet of farmed fish: past and future. Anim Feed Sci Technol 203:1–22. https://doi.org/10.1016/j.anifeedsci.2015.03.001
Hermann BT, Reusch TBH, Hanel R (2016) Effects of dietary purified rapeseed protein concentrate on hepatic gene expression in juvenile turbot (Psetta maxima). Aquac Nutr 22:170–180. https://doi.org/10.1111/anu.12251
Hua K et al (2019) The future of aquatic protein: implications for protein sources in aquaculture diets. One Earth 1:316–329. https://doi.org/10.1016/j.oneear.2019.10.018
Ido A, Hashizume A, Ohta T, Takahashi T, Miura C, Miura T (2019) Replacement of fish meal by defatted yellow mealworm (Tenebrio molitor) larvae in diet improves growth performance and disease resistance in red seabream (Pargus major). Animals 9.https://doi.org/10.3390/ani9030100
Imsland AK, Foss A, Naevdal G, Cross T, Bonga SW, Ham EV, Stefansson SO (2000) Countergradient variation in growth and food conversion efficiency of juvenile turbot. J Fish Biol 57:1213–1226. https://doi.org/10.1006/jfbi.2000.1384
Imsland AK, Foss A, Stefansson SO (2001) Variation in food intake, food conversion efficiency and growth of juvenile turbot from different geographic strains. J Fish Biol 59:449–454. https://doi.org/10.1006/jfbi.2001.1637
Imsland AK, Sunde LM, Folkvord A, Stefansson SO (1996) The interaction of temperature and fish size on growth of juvenile turbot. J Fish Biol 49:926–940. https://doi.org/10.1111/j.1095-8649.1996.tb00090.x
Jia S, Li X, He W, Wu G (2022) Protein-sourced feedstuffs for aquatic animals in nutrition researchand aquaculture. In: Wu G (ed) Recent Advances in Animal Nutrition and Metabolism. Springer International Publishing, Cham, pp 237–261
Jozefiak A, Nogales-Merida S, Mikolajczak Z, Rawski M, Kieronczyk B, Mazurkiewicz J (2019) The utilization of full-fat insect meal in rainbow trout (Oncorhynchus mykiss) nutrition: the effects on growth performance, intestinal microbiota and gastrointestinal tract histomorphology. Annals of Animal Science 19:747–765. https://doi.org/10.2478/aoas-2019-0020
Karapanagiotidis IT, Psofakis P, Mente E, Malandrakis E, Golomazou E (2019) Effect of fishmeal replacement by poultry by-product meal on growth performance, proximate composition, digestive enzyme activity, haematological parameters and gene expression of gilthead seabream (Sparus aurata). Aquac Nutr 25:3–14. https://doi.org/10.1111/anu.12824
Kaushik SJ, Cravedi JP, Lalles JP, Sumpter J, Fauconneau B, Laroche M (1995) Partial or total replacement of fish meal by soybean protein on growth, protein utilization, potential estrogenic or antigenic effects, cholesterolemia and flesh quality in rainbow trout, Oncorhynchus mykiss. Aquaculture 133:257–274. https://doi.org/10.1016/0044-8486(94)00403-B
Keppler D, Decker K. 1988. 1.2 Glycogen in Bergmeyer HU, ed. Methods of enzymatic analysis: metabolites 1: Carbohydrates, vol. VI. Weinheim, Basel: Verlag Chemie.
Kroeckel S, Harjes AGE, Roth I, Katz H, Wuertz S, Susenbeth A, Schulz C (2012) When a turbot catches a fly: evaluation of a pre-pupae meal of the Black Soldier Fly (Hermetia illucens) as fish meal substitute — growth performance and chitin degradation in juvenile turbot (Psetta maxima). Aquaculture 364–365:345–352. https://doi.org/10.1016/j.aquaculture.2012.08.041
Krogdahl A, Sundby A, Olli JJ (2004) Atlantic salmon (Salmo salar) and rainbow trout (Oncorhynchus mykiss) digest and metabolize nutrients differently. Effects of water salinity and dietary starch level. Aquaculture 229:335–360. https://doi.org/10.1016/S0044-8486(03)00396-X
Kumar V, Sinha AK, Makkar HP, De Boeck G, Becker K (2012) Phytate and phytase in fish nutrition. J Anim Physiol Anim Nutr 96:335–364. https://doi.org/10.1111/j.1439-0396.2011.01169.x
Leknes E, Imsland AK, Gustavsson A, Gunnarsson S, Thorarensen H, Arnason J (2012) Optimum feed formulation for turbot, Scophthalmus maximus (Rafinesque, 1810) in the grow-out phase. Aquaculture 344:114–119. https://doi.org/10.1016/j.aquaculture.2012.03.011
Li X, Liu Y, Blancheton JP (2013) Effect of stocking density on performances of juvenile turbot (Scophthalmus maximus) in recirculating aquaculture systems. Chin J Oceanol Limnol 31:514–522. https://doi.org/10.1007/s00343-013-2205-0
Li Y, Kortner TM, Chikwati EM, Belghit I, Lock E-J, Krogdahl Å (2020) Total replacement of fish meal with black soldier fly (Hermetia illucens) larvae meal does not compromise the gut health of Atlantic salmon (Salmo salar). Aquaculture 520.https://doi.org/10.1016/j.aquaculture.2020.734967
Li Z, Bao N, Ren T, Han Y, Jiang Z, Bai Z, Hu Y, Ding J (2019) The effect of a multi-strain probiotic on growth performance, non-specific immune response, and intestinal health of juvenile turbot, Scophthalmus maximus L. Fish Physiol Biochem 45:1393–1407. https://doi.org/10.1007/s10695-019-00635-4
Liu XW, Mai KS, Liufu ZG, Ai QH (2014a) Effects of dietary protein and lipid levels on growth, nutrient utilization, and the whole-body composition of turbot, Scophthalmus maximus, Linnaeus 1758, at different growth stages. J World Aquaculture Soc 45:355–366. https://doi.org/10.1111/jwas.12135
Liu YZ, He G, Wang QC, Mai KS, Xu W, Zhou HH (2014b) Hydroxyproline supplementation on the performances of high plant protein source based diets in turbot (Scophthalmus maximus L.). Aquaculture 433:476–480. https://doi.org/10.1016/j.aquaculture.2014.07.002
Lorentzen M, Maage A, Julshamn K (1996) Manganese supplementation of a practical, fish meal based diet for Atlantic salmon parr. Aquac Nutr 2:121–125. https://doi.org/10.1111/j.1365-2095.1996.tb00019.x
Lu F, Haga Y, Satoh S (2015) Effects of replacing fish meal with rendered animal protein and plant protein sources on growth response, biological indices, and amino acid availability for rainbow trout Oncorhynchus mykiss. Fish Sci 81:95–105. https://doi.org/10.1007/s12562-014-0818-7
Ma R, Hou H, Mai K, Bharadwaj AS, Ji F, Zhang W (2015) Comparative study on the effects of chelated or inorganic manganese in diets containing tricalcium phosphate and phytate on the growth performance and physiological responses of turbot Scophthalmus maximus. Aquac Nutr 21:780–787. https://doi.org/10.1111/anu.12206
Maiolo S, Parisi G, Biondi N, Lunelli F, Tibaldi E, Pastres R (2020) Fishmeal partial substitution within aquafeed formulations: life cycle assessment of four alternative protein sources. Int J Life Cycle Assess 25:1455–1471. https://doi.org/10.1007/s11367-020-01759-z
Matos E, Dias J, Dinis MT, Silva TS (2017) Sustainability vs. quality in gilthead seabream (Sparus aurata L.) farming: are trade-offs inevitable? Rev Aquac 9:388–409. https://doi.org/10.1111/raq.12144
Miao SY, Nie Q, Miao HJ, Zhang WB, Mai KS (2016) Effects of dietary carbohydrate-to-lipid ratio on the growth performance and feed utilization of juvenile turbot (Scophthalmus maximus). Journal of Ocean University of China 15:660–666. https://doi.org/10.1007/s11802-016-2934-8
Milhazes-Cunha H, Otero A (2017) Valorisation of aquaculture effluents with microalgae: the integrated multi-trophic aquaculture concept. Algal Research-Biomass Biofuels and Bioproducts 24:416–424. https://doi.org/10.1016/j.algal.2016.12.011
Nagel F, Appel T, Rohde C, Kroeckel S, Schulz C. 2017. Blue mussel protein concentrate versus prime fish meal protein as a dietary attractant for turbot (Psetta maxima L.) given rapeseed proteinbased diets. Journal of Aquaculture Research & Development s2.https://doi.org/10.4172/2155-9546.S2-012.
Nagel F, von Danwitz A, Tusche K, Kroeckel S, van Bussel CGJ, Schlachter M, Adem H, Tressel R-P, Schulz C (2012) Nutritional evaluation of rapeseed protein isolate as fish meal substitute for juvenile turbot (Psetta maxima L.) — impact on growth performance, body composition, nutrient digestibility and blood physiology. Aquaculture 356–357:357–364. https://doi.org/10.1016/j.aquaculture.2012.04.045
Naylor RL, Hardy RW, Bureau DP, Chiu A, Elliott M, Farrell AP, Forster I, Gatlin DM, Goldburg RJ, Hua K (2009) Feeding aquaculture in an era of finite resources. Proc Natl Acad Sci 106:15103–15110
Naylor RL, Hardy RW, Buschmann AH, Bush SR, Cao L, Klinger DH, Little DC, Lubchenco J, Shumway SE, Troell M (2021) A 20-year retrospective review of global aquaculture. Nature 591:551–563. https://doi.org/10.1038/s41586-021-03308-6
Newton GL, Sheppard DC, Watson DW, Burtle GJ, Dove CR, Tomberlin JK, Thelen EE (2005) The black soldier fly, Hermetia illucens, as a manure management/resource recovery tool. Pages 5–7. Symposium on the state of the science of Animal Manure and Waste Management: Semantic Scholar
Obaldo LG, Divakaran S, Tacon AG (2002) Method for determining the physical stability of shrimp feeds in water. Aquac Res 33:369–377. https://doi.org/10.1046/j.1365-2109.2002.00681.x
Oliva-Teles A, Goncalves P (2001) Partial replacement of fishmeal by brewers yeast (Saccaromyces cerevisae) in diets for sea bass (Dicentrarchus labrax) juveniles. Aquaculture 202:269–278. https://doi.org/10.1016/S0044-8486(01)00777-3
Olsen RL, Hasan MR (2012) A limited supply of fishmeal: impact on future increases in global aquaculture production. Trends Food Sci Technol 27:120–128. https://doi.org/10.1016/j.tifs.2012.06.003
Omnes MH, Silva FCP, Moriceau J, Aguirre P, Kaushik S, Gatesoupe FJ (2015) Influence of lupin and rapeseed meals on the integrity of digestive tract and organs in gilthead seabream (Sparus aurata L.) and goldfish (Carassius auratus L.) juveniles. Aquac Nutr 21:223–233. https://doi.org/10.1111/anu.12162
Øverland M, Sørensen M, Storebakken T, Penn M, Krogdahl Å, Skrede A (2009) Pea protein concentrate substituting fish meal or soybean meal in diets for Atlantic salmon (Salmo salar)—Effect on growth performance, nutrient digestibility, carcass composition, gut health, and physical feed quality. Aquaculture 288:305–311. https://doi.org/10.1016/j.aquaculture.2008.12.012
Pichavant K, Maxime V, Thebault MT, Ollivier H, Garnier JP, Bousquet B, Diouris M, Boeuf G, Nonnotte G (2002) Effects of hypoxia and subsequent recovery on turbot Scophthalmus maximus: hormonal changes and anaerobic metabolism. Mar Ecol Prog Ser 225:275–285. https://doi.org/10.3354/meps225275
Postel L, Fock H, Hagen W (2000) Biomass and abundance. In: Harris R, Wiebe P, Lenz J, Skjoldal HR, Huntley M (eds) ICES Zooplankton Methodology Manual. Academic Press, London, pp 83–192
Read ES, Barrows FT, Gaylord TG, Paterson J, Petersen MK, Sealey WM (2014) Investigation of the effects of dietary protein source on copper and zinc bioavailability in fishmeal and plant-based diets for rainbow trout. Aquaculture 432:97–105. https://doi.org/10.1016/j.aquaculture.2014.04.029
Refstie S, Baeverfjord G, Seim RR, Elvebo O (2010) Effects of dietary yeast cell wall beta-glucans and MOS on performance, gut health, and salmon lice resistance in Atlantic salmon (Salmo salar) fed sunflower and soybean meal. Aquaculture 305:109–116. https://doi.org/10.1016/j.aquaculture.2010.04.005
Refstie S, Sahlstrom S, Brathen E, Baeverfjord G, Krogedal P (2005) Lactic acid fermentation eliminates indigestible carbohydrates and antinutritional factors in soybean meal for Atlantic salmon (Salmo salar). Aquaculture 246:331–345. https://doi.org/10.1016/j.aquaculture.2005.01.001
Regost C, Arzel J, Cardinal M, Robin J, Laroche M, Kaushik SJ (2001) Dietary lipid level, hepatic lipogenesis and flesh quality in turbot (Psetta maxima). Aquaculture 193:291–309. https://doi.org/10.1016/S0044-8486(00)00493-2
Regost C, Arzel J, Kaushik SJ (1999) Partial or total replacement of fish meal by corn gluten meal in diet for turbot (Psetta maxima). Aquaculture 180:99–117. https://doi.org/10.1016/S0044-8486(99)00026-5
Rema P, Saravanan S, Armenjon B, Motte C, Dias J (2019) Graded incorporation of defatted yellow mealworm (Tenebrio molitor) in rainbow trout (Oncorhynchus mykiss) diet improves growth performance and nutrient retention. Animals 9.https://doi.org/10.3390/ani9040187
Robaina L, Izquierdo MS, Moyano FJ, Socorro J, Vergara JM, Montero D, Fernandezpalacios H (1995) Soybean and lupin seed meals as protein sources in diets for gilthead seabream (Sparus aurata): nutritional and histological implications. Aquaculture 130:219–233. https://doi.org/10.1016/0044-8486(94)00225-D
San Martin D, Orive M, Iñarra B, Castelo J, Estévez A, Nazzaro J, Iloro I, Elortza F, Zufía J (2020) Brewers’ spent yeast and grain protein hydrolysates as second-generation feedstuff for aquaculture feed. Waste and Biomass Valorization 11:5307–5320. https://doi.org/10.1007/s12649-020-01145-8
Sevgili H, Kurtoglu A, Oikawa M, Ozturk E, Dedebali N, Emre N, Pak F (2014) High dietary lipids elevate carbon loss without sparing protein in adequate protein-fed juvenile turbot (Psetta maxima). Aquacult Int 22:797–810. https://doi.org/10.1007/s10499-013-9708-8
Siddik MAB, Howieson J, Fotedar R, Partridge GJ (2020) Enzymatic fish protein hydrolysates in finfish aquaculture: a review. Rev Aquac 13:406–430. https://doi.org/10.1111/raq.12481
Silva MS, Krockel S, Prabhu PAJ, Koppe W, Ornsrud R, Waagbo R, Araujo P, Amlund H (2019) Apparent availability of zinc, selenium and manganese as inorganic metal salts or organic forms in plant-based diets for Atlantic salmon (Salmo salar). Aquaculture 503:562–570. https://doi.org/10.1016/j.aquaculture.2019.01.005
Soengas JL, Barciela P, Aldegunde M (1995) Variations in carbohydrate metabolism during gonad maturation in female turbot (Scophthalmus maximus). Mar Biol 123:11–18. https://doi.org/10.1007/Bf00350318
Stadtlander T, Stamer A, Buser A, Wohlfahrt J, Leiber F, Sandrock C (2017) Hermetia illucens meal as fish meal replacement for rainbow trout on farm. J Insects Food Feed 3:165–175. https://doi.org/10.3920/Jiff2016.0056
Storebakken T, Shearer KD, Baeverfjord G, Nielsen BG, Asgard T, Scott T, De Laporte A (2000) Digestibility of macronutrients, energy and amino acids, absorption of elements and absence of intestinal enteritis in Atlantic salmon, Salmo salar, fed diets with wheat gluten. Aquaculture 184:115–132. https://doi.org/10.1016/S0044-8486(99)00316-6
Storebakken T, Shearer KD, Roem AJ (1998) Availability of protein, phosphorus and other elements in fish meal, soy-protein concentrate and phytase-treated soy-protein-concentrate-based diets to Atlantic salmon, Salmo salar. Aquaculture 161:365–379. https://doi.org/10.1016/S0044-8486(97)00284-6
Sugiura ST, Dong FM, Hardy RW (1998) Effects of dietary supplements on the availability of minerals in fish meal; preliminary observations. Aquaculture 160:283–303. https://doi.org/10.1016/S0044-8486(97)00302-5
Tacon AGJ, Hasan MR, Metian M (2011) Demand and supply of feed ingredients for farmed fish and crustaceans: trends and prospects. FAO Fisheries and Aquaculture technical paper:I
Vallejos-Vidal E, Reyes-López F, Teles M, MacKenzie S (2016) The response of fish to immunostimulant diets. Fish Shellfish Immunology 56:34–69. https://doi.org/10.1016/j.fsi.2016.06.028
van Bussel CGJ, Schroeder JP, Mahlmann L, Schulz C (2014) Aquatic accumulation of dietary metals (Fe, Zn, Cu Co, Mn) in recirculating aquaculture systems (RAS) changes body composition but not performance and health of juvenile turbot (Psetta maxima). Aquacult Eng 61:35–42. https://doi.org/10.1016/j.aquaeng.2014.05.003
Van Huis A (2013) Potential of insects as food and feed in assuring food security. Annu Rev Entomol 58:563–583
von Danwitz A, van Bussel CGJ, Klatt SF, Schulz C (2016) Dietary phytase supplementation in rapeseed protein based diets influences growth performance, digestibility and nutrient utilisation in turbot (Psetta maxima L.). Aquaculture 450:405–411. https://doi.org/10.1016/j.aquaculture.2015.07.026
Wan AHL, Davies SJ, Soler-Vila A, Fitzgerald R, Johnson MP (2019) Macroalgae as a sustainable aquafeed ingredient. Rev Aquac 11:458–492. https://doi.org/10.1111/raq.12241
Wang Q, He G, Mai K, Xu W, Zhou H (2016) Fishmeal replacement by mixed plant proteins and maggot meal on growth performance, target of rapamycin signalling and metabolism in juvenile turbot (Scophthalmus maximus L.). Aquac Nutr 22:752–758. https://doi.org/10.1111/anu.12296
Wang Y, Wang F, Ji WX, Han H, Li P (2015) Optimizing dietary protein sources for Japanese sea bass (Lateolabrax japonicus) with an emphasis on using poultry by-product meal to substitute fish meal. Aquac Res 46:874–883. https://doi.org/10.1111/are.12242
Wanka KM, Schulz C, Kloas W, Wuertz S (2019) Administration of host-derived probiotics does not affect utilization of soybean meal enriched diets in juvenile turbot (Scophthalmus maximus). J Appl Ichthyol 35:1004–1015. https://doi.org/10.1111/jai.13929
Weiß M, Buck BH (2017) Partial replacement of fishmeal in diets for turbot (Scophthalmus maximus, Linnaeus, 1758) culture using blue mussel (Mytilus edulis, Linneus, 1758) meat. J Appl Ichthyol 33:354–360. https://doi.org/10.1111/jai.13323
Whiteman KW, Gatlin DM (2005) Evaluation of fisheries by-catch and by-product meals in diets for red drum Sciaenops ocellatus L. Aquac Res 36:1572–1580. https://doi.org/10.1111/j.1365-2109.2005.01380.x
Wu YB, Ren X, Chai XJ, Li P, Wang Y (2018) Replacing fish meal with a blend of poultry by-product meal and feather meal in diets for giant croaker (Nibea japonica). Aquac Nutr 24:1085–1091. https://doi.org/10.1111/anu.12647
Zeng L, Lei JL, Ai CX, Hong WS, Liu B (2015) Protein-sparing effect of carbohydrate in diets for juvenile turbot Scophthalmus maximus reared at different salinities. Chin J Oceanol Limnol 33:57–69. https://doi.org/10.1007/s00343-015-4070-5
Zhang Y, Overland M, Sorensen M, Penn M, Mydland LT, Shearer KD, Storebakken T (2012) Optimal inclusion of lupin and pea protein concentrates in extruded diets for rainbow trout (Oncorhynchus mykiss). Aquaculture 344:100–113. https://doi.org/10.1016/j.aquaculture.2012.03.012
Acknowledgements
The authors would like to thank the technical staff from the marine aquaculture group, the integrated ecophysiology group, the apprentices and the marine geochemistry group at the AWI and the food chemistry lab at the University of Applied Sciences Bremerhaven for their efforts and valuable assistance. Special thanks also to Jorge Dias and the SPAROS aquafeed production team for feed formulation and prototyping.
Funding
Open Access funding enabled and organized by Projekt DEAL. The study was part of the GAIN2020 project funded by the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 773330.
Author information
Authors and Affiliations
Contributions
CH: Conceptualization, methodology, validation, formal analysis, investigation, data curation, writing (original draft), writing (review and editing) and visualisation.
JP: Methodology, validation, investigation and writing (review and editing).
GL: Methodology, validation, formal analysis, resources, writing (review and editing) and supervision.
JJ: Conceptualization, methodology, writing (review and editing), project administration and funding acquisition.
GP: Methodology, resources and writing (review and editing).
LC: Conceptualization, methodology, resources, writing (review and editing), project administration and funding acquisition.
RP: Writing (review and editing), project administration and funding acquisition.
BB: Conceptualization, methodology, writing (review and editing), supervision, project administration and funding acquisition.
Corresponding author
Ethics declarations
Ethics approval
The experiments were performed under the guidelines of the local authority ‘Food surveillance, animal welfare and veterinary service (LMTVet)’ of the state of Bremen with the permission to carry out animal experiments (500–427-103–1/2019–1-19).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Gavin Burnell
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hoerterer, C., Petereit, J., Lannig, G. et al. Sustainable fish feeds: potential of emerging protein sources in diets for juvenile turbot (Scophthalmus maximus) in RAS. Aquacult Int 30, 1481–1504 (2022). https://doi.org/10.1007/s10499-022-00859-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-022-00859-x