Abstract
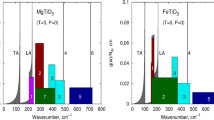
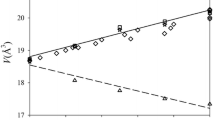
The local structural heterogeneity and energetic properties of 22 natural Mg–Fe cordierites, ideal formula (Mg,Fe)2Al4Si5O18·x(H2O,CO2), were investigated at length scales given by powder infrared spectroscopy (IR) and also by published electronic absorption spectra. The studied samples have iron mole fractions from XFe = 0.06 to 0.82 and cover most of the Mg–Fe cordierite binary. Variations in wavenumbers and line widths of the IR bands were determined as a function of composition. Most modes shift linearly to lower wavenumbers with increasing XFe, except those at high wavenumbers located between 900 and 1,200 cm-1. They are vibrations that have a large internal (Si,Al)O4 character and are not greatly affected by Mg–Fe exchange on the octahedral site. The lower wavenumber modes can be best characterized as lattice vibrations having mixed character. The systematics of the wavenumber shifts suggest small continuous variations in the "average" cordierite structure with Mg–Fe exchange and are consistent with an ideal volume of mixing, ΔVmix= 0, behavior (Boberski and Schreyer 1990). IR line broadening was measured using the autocorrelation function for three wavenumber regions in order to determine the range of structural heterogeneity between roughly 2 and 100 Å (0.2–10.0 nm) in the solid solution. In order to do this, an empirical correction was first made to account for the effect that small amounts of channel Na have on the phonon systematics. The results show that between 1,200 and 540 cm-1 the line widths of the IR bands broaden slightly and linearly with increasing XFe. Between 350 and 125 cm-1 nonlinear behavior was observed and it may be related to dynamic effects. These results suggest minimal excess elastic enthalpies of mixing for Mg–Fe cordierite solid solutions. Channel Na should affect measurably the thermodynamic properties of natural cordierites as evidenced by variations in the IR spectra of Na-containing samples. Occluded H2O (Class I) and CO2 should have little interaction with the framework and can be considered nearly "free" molecules. They should not give rise to measurable structural heterogeneity in the framework. The contribution of the crystal field stabilization energy (CFSE) of octahedral Fe2+ to the energetics of Mg–Fe cordierites was also investigated using published electronic absorption spectra (Khomenko et al. 2001). Two bands are observed between 8,000 and 10,500 cm-1 and they represent electronic dd-excitations of octahedral Fe2+ derived from the 5T2g → 5Eg transition. They shift to higher wavenumbers with increasing XMg in cordierite. An analysis gives slightly asymmetric excess -ΔCFSE across the Mg–Fe cordierite join with a maximum of about −550 J/mole towards iron-rich compositions.












Similar content being viewed by others
References
Armbruster T (1982) Effect of H2O on the structure of low-cordierite, a single crystal X-ray study. Proc 13th IMA General Meeting, Varna, Bulgaria, pp 485–503
Armbruster T (1985) Fe-rich cordierites from acid volcanic rocks, an optical and X-ray single-crystal structure study. Contrib Mineral Petrol 91:180–187
Armbruster T (1986) Role of Na in the structure of low-cordierite: a single-crystal X-ray study. Am Mineral 71:746–757
Armbruster T, Irouschek A (1983) Cordierites from the Lepontine Alps: Na + Be → Al substitution, gas content, cell parameters, and optics. Am Mineral 82:389-396
Atkinson AJ, Carpenter MA, Salje EKH (1999) Hard mode infrared spectroscopy of plagioclase feldspars. Eur J Mineral 11:7–23
Boberski C, Schreyer W (1990) Synthesis and water contents of Fe2+-bearing cordierites. Eur J Mineral 2:565–584
Boffa Ballaran T, Carpenter MA, Domeneghetti MC, Salje EKH, Tazzoli V (1998) Structural mechanisms of solid solution and cation ordering in augite–jadeite pyroxenes. II: a microscopic perspective. Am Mineral 83:434–443
Boffa Ballaran T, Carpenter MA, Geiger CA, Koziol AM (1999) Local structural heterogeneity in garnet solid solutions. Phys Chem Miner 26:554–569
Burns RG (1993) Mineralogical applications of crystal field theory. Cambridge University Press, New York
Carpenter MA, Boffa Ballaran T (2001) The influence of elastic strain heterogeneities in silicate solid solutions. In: Geiger CA (ed) Solid solutions in silicate and oxide systems. European Notes in Mineralogy, vol 3, pp 155–178
Cerny P, Povondra P (1966) Beryllian cordierite from Vezna: (Na, K) + Be → Al. Neues Jahrb Mineral Monatsh 36–44
Chatillon-Colinet C, Newton RC, Perkins III D, Kleppa OJ (1983) Thermochemistry of (Fe2+,Mg)SiO3 orthopyroxene. Geochim Cosmochim Acta 47:1597–1603
Ferreira LG, Mbaye AA, Zunger A (1988) Chemical and elastic effects on isostructural phase diagrams: the ε-G approach. Phys Rev B 37:10547–10570
Fuchs, LH (1969) Occurrence of cordierite and aluminous orthoenstatite in the Allende meteorite. Am Mineral 54:1645–1653
Geiger CA (2001) Thermodynamic mixing properties of binary oxide and silicate solid solutions determined by direct measurements: the role of strain. In: Geiger CA (ed) Solid solutions in silicate and oxide systems. European Notes in Mineralogy, vol 3, pp 71–100
Geiger CA, Kolesov BA (2003) Microscopic–macroscopic relationships in silicates: examples from IR and Raman spectroscopy and heat capacity measurements. In: Gramaccioli CM (ed) Energy modelling in minerals. European Notes in Mineralogy, vol 4, pp 347–387
Geiger CA, Voigtländer H (2000) The heat capacity of synthetic anhydrous Mg and Fe cordierite. Contrib Mineral Petrol 138:46–50
Geiger CA, Newton RC, Kleppa OJ (1987) Enthalpy of mixing of synthetic almandine-grossular and almandine-pyrope garnets from high-temperature solution calorimetry. Geochim Cosmochim Acta 51:1755–1763
Geiger CA, Winkler B, Langer K (1989) Infrared spectra of synthetic almandine-grossular and almandine-pyrope garnet solid solutions: evidence for equivalent site behavior. Mineral Mag 53:231–237
Geiger CA, Armbruster T, Khomenko V, Quartieri S (2000a) Cordierite I: the coordination of Fe2+. Am Mineral 85:1255–1264
Geiger CA, Rager H, Czank M (2000b) Cordierite III: the coordination and concentration of Fe3+. Contrib Mineral Petrol 140:344–352
Gibbs GV (1966) The polymorphism in cordierite I: the crystal structure of low cordierite. Am Mineral 51:1068–1087
Greenwood HJ (1979) Some linear and non-linear problems in petrology. Geochim Cosmochim Acta 43:1873–1885
Güttler B, Salje E, Putnis A (1989) Structural states of Mg cordierite III: infrared spectroscopy and the nature of the hexagonal-modulated transition. Phys Chem Miner 16:365–373
Hammonds KD, Dove MT, Giddy AP, Heine V, Winkler B (1996) Rigid-unit modes and structural phase transitions in framework silicates. Am Mineral 81:1057–1079
Hammonds KD, Bosenick A, Dove MT, Heine V (1998) Rigid unit modes in crystal structures with octahedrally coordinated atoms. Am Mineral 83:476–479
Hochella MF Jr, Brown GE Jr, Ross FK, Gibbs GV (1979) High-temperature crystal chemistry of hydrous Mg- and Fe-cordierite. Am Mineral 64:337–351
Kerrick DM, Darken LS (1975) Statistical thermodynamic models for ideal oxide and silicate solid solutions, with application to plagioclase. Geochim Cosmochim Acta 39:1431–1442
Khomenko V, Langer K, Geiger CA (2001) Structural allocation of iron ions in cordierite: spectroscopic study. Contrib Mineral Petrol 141:381–396
Kojitani H, Akaogi M (1994) Calorimetric study of olivine solid solutions in the system Mg2SiO4–Fe2SiO4. Phys Chem Miner 20:536–540
Kolesov BA, Geiger CA (2000) Cordierite II: the role of H2O and CO2. Am Mineral 85:1265–1274
Langer K, Schreyer W (1969) Infrared and powder x-ray diffraction studies on the polymorphism of cordierite, Mg2(Al4Si5O18). Am Mineral 54:1442–1459
Langer K, Schreyer W (1976) Apparent effects of molecular water on the lattice geometry of cordierite: a discussion. Am Mineral 61:1036–1040
Malcherek T, Domeneghetti MC, Tazzoli V, Ottolini L, McCammon C, Carpenter MA (2001) Structural properties of ferromagnesian cordierites. Am Mineral 86:66–79
Miyashiro A, Iiyama JT (1954) A preliminary note on a new mineral, indialite, polymorphic with cordierite. Imp Acad Jpn Proc 30:746–751
Mueller RF (1962) Energetics of certain silicate solid solutions. Geochim Cosmochim Acta 26:581–598
Newton RC (1966) BeO in pegmatitic cordierite. Mineral Mag 35:920–927
Newton RC, Charlu TV, Kleppa OJ (1977) Thermochemistry of high pressure garnets and clinopyroxenes in the system CaO–MgO–Al2O3–SiO2. Geochim Cosmochim Acta 41:369–377
Pattison DRM, Spear FS, Debuhr CL, Cheney JT, Guidotti CV (2002) Thermodynamic modelling of the reaction muscovite + cordierite → Al2SiO5 + biotite + quartz + H2O: constraints from natural assemblages and implications for the metapelitic petrogenetic grid. J Metamorph Geol 20:99–118
Pryce MW (1973) Low-iron cordierite in phlogopite schist from White Well, Western Australia. Mineral Mag 39:241–243
Salje EKH, Bismayer U (1997) Hard mode spectroscopy: the concept and applications. Phase Trans 63:1–75
Salje EKH, Carpenter MA, Malcherek T, Boffa Ballaran T (2000) Autocorrelation analysis of infrared spectra of minerals. Eur J Mineral 12:503–519
Schreyer W (1985) Experimental studies on cation substitutions and fluid incorporation in cordierite. Bull Mineral 108:273–291
Schreyer W, Maresch WV, Daniels P, Wolfsdorff P (1990) Potassic cordierites: characteristic minerals for high-temperature, very low pressure environments. Contrib Mineral Petrol 105:162–172
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst A32:751–767
Srivastava GP, Martins JL, Zunger A (1985) Atomic structure and ordering in semiconductor alloys. Phys Rev B 31:2561–2564
Stanek J, Miskovsky J (1964) Iron-rich cordierite from the Dolni Bory pegmatite (in Czech). Cas Mineral Geol 9:191–192
Tarantino SC, Boffa Ballaran T, Carpenter MA, Domeneghetti MC, Tazzoli V (2002) Mixing properties of the enstatite–ferrosilite solid solution: II. A microscopic perspective. Eur J Mineral 14:537–547
Thompson AB (1976) Mineral reactions in pelitic rocks: I. Prediction of P–T–X (Fe–Mg) phase relations. Am J Sci 276:401–424
Wallace H, Wenk HR (1980) Structure variation in low cordierites. Am Mineral 65:96–111
White WB (1974) Order–disorder effects. In: Farmer VC (ed) The infrared spectra of minerals. Mineral Soc Great Britain, pp 87–110
Winkler B, Milman V, Payne MC (1994) Orientation, location, and total energy of hydration of channel H2O in cordierite investigated by ab-initio total energy calculations. Am Mineral 79:200–204
Wood BJ, Kleppa OJ (1981) Thermochemistry of forsterite–fayalite olivine solid solutions. Geochim Cosmochim Acta 45:529–534
Wood BJ, Holland TJB, Newton RC, Kleppa OJ (1980) Thermochemistry of jadeite–diopside pyroxenes. Geochim Cosmochim Acta 44:1363–1371
Zhang M, Wruck B, Graeme Barber A, Salje EKH, Carpenter MA (1996) Phonon spectra of alkali feldspars: phase transitions and solid solutions. Am Mineral 81:92–104
Acknowledgements
We thank Tiziana Boffa Ballaran, Michael A. Carpenter, and Eckhard Salje for showing us the fine points of HMIRS. J. Vry, Th. Armbruster, A. Speer, P. Raase, and V. Khomenko generously provided cordierite samples. This work was supported by a grant, Ge 659/6-2, from the Deutsche Forschungsgemeinschaft. The comments of two anonymous reviewers and D.R.M. Pattison led to changes that improved significantly the manuscript. Professor J. Hoefs is thanked for his help in the review process.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: J. Hoefs
Rights and permissions
About this article
Cite this article
Geiger, C.A., Grams, M. Cordierite IV: structural heterogeneity and energetics of Mg–Fe solid solutions. Contrib Mineral Petrol 145, 752–764 (2003). https://doi.org/10.1007/s00410-003-0478-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00410-003-0478-6