Abstract
Over the past 40 years, the significance of microzooplankton grazing in oceanic carbon cycling has been highlighted with the help of dilution experiments. The ecologically relevant Western Antarctic Peninsula (WAP) ecosystem in the Southern Ocean (SO), however, has not been well studied. Here we present data from dilution experiments, performed at three stations around the northern tip of the WAP to determine grazing rates of small zooplankton (hetero- and mixotrophic members of the 0.2–200 µm size fraction, SZP) on auto- and heterotrophic members of the < 200 µm plankton community as well as their gross growth. While variable impacts of SZP grazing on carbon cycling were measured, particulate organic carbon, not the traditionally used parameter chlorophyll a, provided the best interpretable results. Our results suggested that heterotrophic picoplankton played a significant role in the carbon turnover at all stations. Finally, a comparison of two stations with diverging characteristics highlights that SZP grazing eliminated 56–119% of gross particulate organic carbon production from the particulate fraction. Thus, SZP grazing eliminated 20–50 times more carbon from the particulate fraction compared to what was exported to depth, therefore significantly affecting the efficiency of the biological carbon pump at these SO sites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In vast areas of the Southern Ocean (SO) phytoplankton growth is controlled by ‘bottom up’ factors such as trace metal concentrations (mainly iron), and/or light availability (Martin et al. 1990; Boyd 2002). Top-down regulation by micro- and nanozooplankton grazing on the plankton community, has not received much attention (Tsuda and Kawaguchi 1997; Garzio et al. 2013). However, both factors crucially define the fate of photosynthetically fixed carbon (~50 Pg annually in the world ocean (Field et al. 1998)), which can have large implications on global carbon cycling. In contrast to phytoplankton cells sedimenting directly to depth, the majority of global primary production (PP) is remineralized between 0 and 200 m depth (Henson et al. 2012), often facilitated by heterotrophic grazers. Consumption of PP occurs either via short, efficient food chains (Ryther 1969), resulting in fecal pellets or carcasses which export a fraction of the originally bound carbon to depth (Turner 2015), or, conversely, the PP is recycled and respired to dissolved organic and inorganic carbon in the microbial loop (Azam et al. 1983; Calbet and Landry 2004; Turner 2015). The composition and activity of the microbial loop community thus has a large influence on the sequestration potential of the greenhouse gas CO2 in the ocean (McNair et al. 2021).
The microplankton is a diverse community (Steinberg and Landry 2017) and ranges in size from 20 to 200 µm (Dussart 1965), which includes herbivorous protists (Paffenhöfer 1998) such as ciliates and dinoflagellates (Sherr and Sherr 2002). Microzooplankton grazing constitutes the largest phytoplankton biomass sink in the oceans (Calbet and Landry 2004; Schmoker et al. 2013), exceeding in numbers larger mesozooplankton like copepods (Calbet and Landry 2004; Löder et al. 2011). While in the North Sea grazing by microzooplankton can occasionally exceed PP rates and thus reduces the standing stock biomass of phytoplankton (Löder et al. 2011), in polar waters the median percentage of PP grazed by microzooplankton is 53–57% (Schmoker et al. 2013). Hence, assuming a respiratory cost of ~50% (Calbet and Landry 2004), microzooplankton in polar waters can reduce carbon export to depth by 26.5–28.5%, even after only one trophic transfer (Calbet and Landry 2004; Schmoker et al. 2013). In addition to its daily consumption of PP, microzooplankton organisms also provide food for larger heterotrophs by secondary production, partially sustained by feeding on particle sizes too small for the former to graze upon (Berk et al. 1977; Calbet and Saiz 2005; Schmidt et al. 2006). Thus, selective grazing of dominant microzooplankton species can influence the ecosystem by allowing less-grazed phytoplankton species to bloom (Löder et al. 2011).
One approach to estimate the impacts of microzooplankton grazing in the field is the dilution technique (Landry and Hassett 1982). This approach assumes that for small protists, even motile ones, grazing is governed by the encounter rates between predator and prey. This is due to the fact that small plankton operates at low Reynolds numbers and the forces of viscosity dominate over those of inertia (Orchard et al. 2016). Thus, if the encounter rate between predator and prey is reduced via serial dilution, less grazing will occur. This method is not undisputed. Results deviating from the typical linear regression with a negative slope are difficult to interpret, and may occur due to selective feeding, saturated grazers, complex nutrient recycling, mixotrophy, toxic substances or trophic cascades inside the incubation bottles (Teixeira and Figueiras 2009; Calbet et al. 2011, 2012; Calbet and Saiz 2013). Nonetheless, it represents the best approach for estimating microzooplankton grazing and gross growth rates available. Since its first publication in 1982, numerous dilution studies have been conducted worldwide, but only 16 studies were conducted in the SO, of which only three papers (Burkill et al. 1995; Tsuda and Kawaguchi 1997; Garzio et al. 2013) and one dissertation (Price 2012) focused on the Western Antarctic Peninsula (WAP) region. Even though the latter four studies agree that microzooplankton is an important sink for primary and secondary production at the WAP, large fluctuations between sampling sites exist. The WAP is one of the most biologically diverse and productive parts of Antarctica (Meredith et al. 2017) and supports seasonally high PP (Steinberg et al. 2012) and large numbers of grazers like krill and salps, as well as top predators like seabirds and marine mammals (Ducklow et al. 2007; Steinberg et al. 2012). Additionally, with a 3.4–5.7 °C increase in mean annual air temperature per century (Vaughan et al. 2003) the WAP is one of the fastest warming regions on the planet and this change is expected to influence the dynamics of zooplankton predator/prey relationships (Steinberg and Landry 2017). Böckmann et al. 2021 for example, described how a shift from krill to salp dominance already being observed in the SO (Atkinson et al. 2004, 2019) affects the bioavailability of the essential trace metal iron to phytoplankton and thus influences PP (Böckmann et al. 2021). The WAP’s ecological relevance and climatic vulnerability, combined with the lack of data on microzooplankton grazing (Price 2012), necessitates a closer investigation of microzooplankton grazing and its role in the biological pump. While former dilution studies presented grazing rates as solely from microzooplankton, grazing by nanozooplankton indisputably also takes place (Agis et al. 2007). Hence, this study speaks of ‘small zooplankton’ (SZP) comprising all heterotrophic and mixotrophic organisms from 0.2 to 200 µm, as opposed to microzooplankton, defined as 20–200 µm.
The objective of this study was to elucidate the importance of SZP grazing in the carbon cycling of the upper water column at three locations around the WAP. While previous studies relied on chlorophyll a (Chl a) as a measure of phytoplankton biomass, this study investigated SZP grazing on a wide array of parameters and plankton groups, including particulate organic carbon (POC), particulate organic nitrogen (PON) and cell abundances of heterotrophic bacteria as well as autotrophic pico- and nanoeukaryotes (PE and NE respectively).
Material and Methods
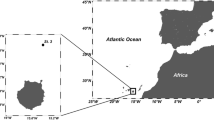
This study was performed in the framework of the POSER project (Population shift and ecosystem response—krill vs. salps), aboard RV Polarstern (PS112) between March and May 2018. Dilution experiments were performed at stations in the Drake Passage (PS112_26: LAT − 62°14.19|LONG − 64°33.44), Scotia Sea (PS112_61: LAT − 60°41.80|LONG − 54°34.25) and Bransfield Strait (PS112_106: LAT − 61°59.25|LONG − 53°59.37; Fig. 1), hereafter referred to as 26, 61 and 106 respectively. Using a polyethylene line connected to an ALMATEC membrane pump, Antarctic seawater was sampled carefully (laminar flow, 3–6 L min−1, bubble free bottle filling) using trace metal clean techniques, successfully used since 2014 (Cabanes et al. 2020; Böckmann et al. 2021; Balaguer et al. 2022). Microscopic analysis of the collected water was conducted to ensure that the plankton community was intact. Water was sampled from a depth of 25 m at stations 26, 61 and 106 near the WAP (Fig. 1) at a surface temperature of 1.2 °C, 1.4 °C and 0.9 °C respectively. The seawater was pumped directly into a trace metal free laboratory container. The seawater was then filtered through a 200 µm mesh in order to exclude larger mesozooplankton grazers as described in Balaguer et al. 2022.
Experimental setup
At each sampling site, initial samples were taken to characterize the ambient plankton community, including POC, PON, Chl a, abundance of autotrophic and heterotrophic pico- and nanoplankton and macronutrients. A serial dilution was then set up by filling triplicate 2 L polycarbonate bottles with either 100% whole seawater (1.0 dilution), or diluting them to 75, 50 and 25% whole seawater (0.75, 0.5 and 0.25 dilution respectively) with 0.2 µm filtered seawater (Acropak capsule, PALL) from the same station. All bottles were incubated in front of full spectrum growth light set to a light intensity of 30 µmol photons m−2 s−1 under a light–dark cycle of 10:14 h at 2 °C, mimicking in situ conditions. The incubations lasted three days (Caron et al. 2000; Garzio et al. 2013), during which the bottles were mixed by gentle turning at least once per day. The experiments were terminated by sampling for plankton community composition, including POC, PON, Chl a, and macronutrients.
POC & PON
POC and PON were sampled, by filtering whole water (POCall and PONall) and 2 µm filtrate (POC<2 µm and PON<2 µm) onto precombusted (15 h, 500 °C) glass fiber filters (GF/F, pore size ∼0.6 μm, Whatman). The filters were stored at − 20 °C and dried for > 12 h at 60 °C prior to sample preparation. The analysis was performed using a Euro Vector CHNS-O elemental analyzer (Euro Elemental Analyzer 3000, HEKAtech GmbH, Wegberg, Germany) and by utilization of the Callidus 5.1 software. Contents of POC and PON were corrected for blank measurements and normalized to filtered volume.
Chl a and PP
Chl a samples were collected by filtering samples onto glass fiber filters (GF/F, pore size ∼0.6 μm, Whatman). These filters were stored frozen until subsequent analysis via standard fluorometric methods (Welschmeyer 1994) on a Trilogy Fluorometer (Turner Designs) using the non-acidification module. PP measurements were carried out according to Koch et al. 2011. Briefly: 0.37 MBq of 14C-bicarbonate (Perkin Elmer) was added to triplicate bottles and incubated in an on deck incubator, allowing for ambient temperature and light conditions. Incubations were terminated after 24 h by filtering up to 100 mL from each bottle onto 0.2 µm pore size polycarbonate filters. At the beginning and end of the incubation, a 250 µL aliquot of each bottle was removed to quantify total activity. After degassing the filters to remove any left over 14C-bicarbonate and adding scintillation cocktail, samples were measured with a scintillation counter (PackardCarb2100TR) and rates calculated according to JGOFS (UNESCO 1994).
Abundance of autotrophic and heterotrophic pico- and nanoplankton
Light microscopy samples (200 mL unfiltered seawater) were taken to qualitatively determine the initial microplankton community. The samples were preserved with Lugol (1% final concentration). Preserved samples were stored at 4 °C in the dark until further analysis by inverted light microscopy (Axio Observer.D1 microscope, Zeiss). After transfer of an appropriate amount of sample (volume based on the present Chl a at the different stations) into the Hydrobios sedimentation chambers for the three stations (50 mL, 10 mL and 25 mL for stations 26, 61 and 106, respectively) and settling of the cells for 24 h, taxonomic groups were enumerated according to the method of Utermöhl, following the recommendations of Edler (Utermöhl 1958; Edler 1979).
Samples for flow cytometric determination of heterotrophic- and cyanobacteria, as well as nano- and picoeukaryotes were taken at each station and from each treatment bottle, fixed with phosphate buffered formalin (final concentration 1%), shock frozen in liquid nitrogen and stored in the dark at − 80 °C. For measurement on a BD Accuri™ C6 Flow Cytometer, (Becton Dickinson) samples were defrosted in a water bath of ambient temperature (20 °C) and 2.11 µm rainbow fluorescent glass beads (Spherotech) were added to each sample. Fluorescence was induced by laser excitations at 488 nm and 640 nm and light emissions were detected in the following four wavelengths: FL1 533/30 nm, FL2 585/40 nm, FL3 > 670 nm, FL4 675/25 nm. In a first run, autofluorescent nano- and picoeukaryotes as well as cyanobacteria were determined. Afterwards, 2 µL of SYBR™ Green II solution (ThermoFisher) was added, samples were allowed to incubate for 10 min in a dark fridge and were then analyzed a second time for abundance of heterotrophic bacteria. The analysis of the results was performed using the BD Accuri C6 software and resulted in the characterization of 4 populations of autotrophic picoeukaryotes < 2 µm, which were differentiated by increasing size (PE 1–4) and one population of autotrophic nanoeukaryotes (> 2 µm, NE), which represent a subset of all nanoflagellates in the size spectrum of 2–5 µm, counted under the microscope. Cyanobacteria as well as high- and low DNA containing heterotrophic bacteria (Gasol et al. 1999) (HDNA bacteria and LDNA bacteria, respectively) were also characterized and counted. Additionally, two group parameters were calculated from these data: bacteria all and PE all.
Dissolved inorganic nutrients
Samples for inorganic macronutrients were taken by filtering 10 mL samples from all treatments through a 0.2 µm syringe filter (ThermoFisher Scientific) into a 15 mL polyethylene sample vial (Falcon). Samples were stored at − 20 °C until analysis. The inorganic nutrients were measured on an Evolution III autoanalyser (Alliance Instruments GmbH, Salzburg, Austria). Methods were modified after Grasshoff et al., 1999 and manufacturer’s instructions (Seal Analytical, https://seal-analytical.com, accessed June 27, 2019) (Grasshoff et al. 1999). Ammonium was determined with a slightly modified method after Holmes et al. 1999.
Definition of terms
Coefficients that are measured/calculated during dilution experiments have been assigned multiple and sometimes confusing abbreviations in the existing body of literature. To avoid confusion, we have compiled all definitions in the supplementary material (Online resource 1). Particularly, we define the term ‘gross absolute POC production’ as the rate of gross POC increase per day. In comparison to PP, which is autotrophic POC production from dissolved inorganic carbon, gross absolute POC production comprises all POC production, including the POC production of heterotrophic organisms from dissolved organic carbon (DOC). Furthermore, by using the term small zooplankton (SZP, 0.2–200 µm) grazing instead of the commonly used microzooplankton grazing, we acknowledge that inside dilution experiments, grazers of both, the nanoplankton and the microplankton, exert grazing pressure on the community (Agis et al. 2007).
Data calculation and statistics
Apparent growth rates (r) for all 12 defined groups were calculated for each parameter and each individual bottle using Eq. 1 from Landry and Hassett 1982:
where t is the time of the incubation in days, fd is the dilution factor in decimal writing, Pt is the value of a given measured parameter after the incubation, and P0 is the respective initial value of a given parameter, before the incubation. A simple linear regression (Altman and Krzywinski 2015) was performed, in which the deviation of the regression’s slope (grazing mortality, g) from 0 was tested at the p = 0.050 significance threshold with the r-values of the respective experiments plotted against the decimal dilution factors. 95% confidence bands, grazing induced mortality rate (g) and the gross growth rate of the respective parameter (k = y-intercept), as well as their respective uncertainties were calculated using SigmaPlot (Systat Software GmbH) and plotted into each graph. Normality statistics for sample sizes < 5000 (Shapiro–Wilk) as well as constant variance tests were performed at the p = 0.050 significance threshold. All published data passed the tests. Net growth rates (µ) in the ambient seawater of all parameters yielding significant regressions were calculated by subtracting the grazing mortality (g) from the gross growth rate (k) with standard errors (SE) propagated.
Absolute rates for parameters with determinable k, g and µ (kabs, gabs, and µabs) were determined by using Eqs. 2, 3 and 4
where P0 is the respective initial value of a given parameter and t is the smallest calculable time interval in days (= 10–12 d, Microsoft Excel). t was chosen to be so small, because in exponential functions the slope increases with the increasing independent variable. Since formula 2 assumes a system in which no grazing occurs and formula 3 assumes a system in which no growth occurs, the errors of both models increase with increasing t. Hence, t is chosen small to obtain realistic kabs, gabs and µabs for a very short time interval, which is then multiplied with 1/t to calculate a close approximation of the true value for the period of 1 day in order to achieve comparability with other sources. Residence times of the parameters were determined by dividing the initial pool by the gabs with standard deviations propagated.
Results
Initial plankton communities
Dissolved inorganic macronutrient concentrations were high throughout the study region, indicative of HNLC waters (Table 1). Dissolved phosphate (PO4−) and dissolved inorganic nitrogen (NOx) were close to Redfield ratios (Redfield et al. 1963; Martiny et al. 2014) at all stations and throughout all dilution steps (Table 1). The biggest differences were detected in dissolved silicate, with concentrations at stations 61 and 106 being ~ 2 and 3 times higher than at station 26, respectively. While particulate organic carbon measured for the entire community (POCall) was highest at station 26, followed by stations 61 and 106 (Table 1), the community’s particulate organic nitrogen (PONall) was similar at stations 26 and 61 and markedly higher than at station 106 (Table 1). This resulted in C:Nall ratios almost matching the Redfield ratio at station 61 (6.4 mol:mol) and exceeding this ratio at stations 26 and 106 (7.8 and 9.4 mol:mol, respectively). Interestingly, the C:N ratio of the < 2 µm size fraction of the plankton (C:N<2 µm) was markedly higher than the community’s (C:Nall) at all three stations (Table 1). In contrast to POC and PON, the Chl a was lowest at station 26, followed by 106 and highest at 61 (Table 1) resulting in POC:Chl a ratios which were lower at stations 61 and 106 than at 26 (Table 1).
At all 3 stations the nanoplankton community (2–20 µm) was dominated numerically by nanoflagellates (2–5 µm), with this group contributing > 80% of the total cells > 2 µm at all stations (Table 1). Low numbers of NE, identified by their Chl a fluorescence, in comparison to the absolute number of nanoflagellates in the size range of 2–5 µm counted under the microscope at a magnification of 640x (both shown in Table 1) suggested an abundant community of heterotrophic nanoflagellates (data accessible via PANGAEA). The total number of heterotrophic bacteria was highest at station 61 and lowest at 106. PE were most abundant at station 61 (Table 1). PP was highest at station 61 and lowest at station 26 (Table 1).
This study focuses on POC since using this parameter constitutes a novel approach for dilution experiments and measurements of this parameter were more reliable and consistent compared to Chl a. Moreover, calculated POC net growth from the dilution series were nearly identical with net growth in the 100% bottles, which represented in situ conditions. To stress the value of the dataset, the parameters Chl a, PON and bacterial numbers were closely examined focusing on their consistency with the POC values. Results of PE and NE, wherever their apparent growth rates were significantly correlated to the dilution factor, are listed in the supplements (Online resource 2).
Growth and grazing mortality
At least three or more groups/parameters at all three stations responded to the dilution treatment and are shown in Table 2 and online resource 2, meaning that a significant regression (p < 0.050) between the decimal dilution factor and the apparent growth rate (r), of a parameter was observed. At station 26, 83% of the 12 parameters showed a significant response to SZP grazing, while at stations 61 and 106 fewer parameters were affected (25% and 50%, respectively). In addition, the net growth rate (µ), calculated from the grazer induced mortality rate (g) and the gross growth rate (k) of each parameter matched the measured apparent growth rate (r) in the raw, undiluted seawater (1.0 dilution) bottles, validating our calculations (Table 2 and online resource 2). Due to low concentrations of Chl a in the 0.5 bottles of station 106, this treatment was excluded from the dataset.
POC & PON
Concentrations of POC and PON integrate all autotrophic and heterotrophic organisms > 0.6 µm. The apparent growth rate of POC (rPOC all) was significantly correlated to the different dilutions at each station. At station 26, the grazer induced mortality of POC (gPOC all) was higher than the gross growth rate (kPOC all) yielding a negative net growth of POC (µPOC all). At stations 61 and 106, the kPOC all was higher than the gPOC all resulting in positive µPOC all. It is noteworthy that the kPOC all was similar at all stations (overlapping standard errors), but at 26 the gPOC all was almost twice as high as at the other two stations. The rPON all was only correlated significantly to the dilution at station 26, yielding here a kPON all and gPON all that resulted in a negative µPON all (Table 2). Direct comparison of k and g for PONall of station 26 with the other two locations is not possible, since the dilution experiments did not yield significant regressions for the measured rPON all values with the dilution factor at 61 and 106. Most interestingly the C:Nall ratios in all experiments significantly (n = 31, p < 0.0001) increased with increasing dilution, resulting in > 100% higher ratios than in the initials (Fig. 2).
The change of the final, relative to the initial C:Nall ratio versus the decimal dilution factor. Data shown was pooled from all 3 experiments. 1.0 corresponds to undiluted seawater. The regression (solid line) was highly significant (n = 31, p < 0.0001). Dashed lines indicate 95% confidence intervals. An increase of C:Nall ratios with increasing dilution of > 100% (right to left) was observed. Please note that the x-axis terminates at 0.2
Chl a
Chl a values integrate all autotrophic organisms > 0.2 μm present in the incubation bottles, including pico- nano- and microphytoplankton. The rChl a values were correlated significantly to the dilutions at stations 26 and 106 (Table 2). At both stations, the kChl a and gChl a were similar (overlapping SE) but resulted in positive µChl a.
Bacteria
rLDNA bacteria values showed a significant correlation to the dilution factor at all stations, while rHDNA bacteria values were only significantly correlated to the dilution factor at stations 26 and 106 (Table 2, Fig. 3b and f). At station 26, both k and g were higher for LDNA bacteria compared to HDNA bacteria. The kbacteria all ranged from 0.34 ± 0.02 at station 106 to 0.54 ± 0.04 at station 26. The gbacteria all, however, was much higher at station 26 in comparison to the other two stations. This resulted in a very low, but still positive µbacteria all at station 26 (Table 2) in comparison to 6 and 4 times higher µbacteria all at 61 and 106, respectively.
Result of dilution series at the three stations 26 (a, b), 61 (c, d) and 106 (e, f) for low DNA (LDNA) and high DNA (HDNA) bacteria. Apparent growth rates (r) on abscissa, dilution factor on ordinate. 1.0 corresponds to undiluted seawater. Slope = grazer induced mortality (g), y-intercept = gross growth rate (k). Significant results obtained for LDNA bacteria at all stations and for HDNA bacteria at stations 26 and 106. Solid lines signify regression, dotted lines 95% confidence bands. Respective n values given in Table 2
Discussion
The dilution method has previously been applied successfully in numerous studies (Schmoker et al. 2013) and proven to be a powerful tool for estimating SZP grazing in aquatic systems. A significant negative correlation (p < 0.050) between the apparent growth rate (r) and the dilution factor is accepted as an ‘interpretable result’. This implies that during the experiment, grazing and growth on the respective parameters occurred in the incubation bottles and that the increase of growth due to a decrease of grazing was proportional to the dilution. Criticism on the method (Calbet and Saiz 2013) mostly revolves around challenging results such as graph shapes and the underestimation of the SZP grazing impact (Calbet et al. 2011). In contrast to this reasonable criticism, this study’s results suggest a dominant SZP grazing impact at all investigated stations (Table 2 Fig. 3).
Three contrasting stations
During this study, the investigated parameters responded very differently to the dilutions at the 3 stations. At off-shelf station 26, a strong grazing pressure of SZP, particularly on heterotrophic organisms was observed, due to i) the high number of parameters (83%) resulting in significant regressions, ii) the 2 times higher gPOC at station 26 in comparison to the other 2 stations (Table 2), iii) the losses from the initial samples relative to the undiluted seawater (1.0 dilution, data accessible via PANGAEA) after the experiment and iv) the 8 times higher gPOC than gChl a (Table 2).
Compared to 26, at station 61, SZP grazing played only a minor role, since only 25% of all measured and calculated parameters resulted in significant regressions (Table 2), although growth of several parameters was observed via an increase in the undiluted seawater bottles (1.0 dilution) compared to the initials. Though weaker than at the other two stations, SZP grazing still accounted for > 50% of POC loss (Fig. 4b) at station 61, possibly due to the disproportionately strong grazing pressure on the bacterial population (Table 2). Like at the other 2 stations, the gLDNA was the highest measured g among all parameters at station 61.
Budget for particulate organic carbon (POC) integrated over the upper 100 m of the water column at station 26 (A) and 61 (B). Carbon (C), dissolved organic carbon (DOC), dissolved inorganic carbon (DIC). All numbers are given as means ± standard deviation and in mg C m−2 d−1. Percentages reflect the share in gross absolute POC production rates. At station 26 more than the gross absolute POC production rate was lost by microzooplankton grazing hence, the POC standing stock was diminished and no POC production remained for export to depth. At station 61, microzooplankton still constituted the biggest loss term of gross absolute POC production in the water column, but after microzooplankton grazing, a net absolute POC production remained. Only ~ 3% of the original gross absolute POC production was lost from the upper 100 m by sedimentation, mostly in form of krill and salp fecal pellets (Pauli et al. 2021a). The lion’s share of net absolute POC production therefore must have remained in the upper 100 m, likely either due to consumption and recycling, or as an addition to the POC standing stock
At the on-shelf station 106, SZP grazing had a moderate influence on the plankton community, with 50% of measured and calculated parameters yielding significant regressions with the dilution factor. Similar to station 26, a higher µPOC than µChl a was observed, suggesting an important contribution of heterotrophic organisms to POCall. In line with this observation and similar to station 26, the LDNA bacteria were the main drivers of the bacterial turnover and carbon cycling at station 106. Overall 66% of the gross absolute POC production was eliminated from the particulate pool by SZP grazing at station 106.
SZP grazing reduced C:N of particulate organic matter
A relieve of SZP grazing pressure resulted in a highly significant increase of the C:Nall ratio of the total particulate organic matter (integrating living cells and dead particles) of > 100% (Fig. 2). Such a change could be the result of either an increase of POC by growth, or a decrease of PON by remineralization. A remineralization of PON, however, would not be dependent on the dilution factor, since it would likely be happening to the particulate organic matter in all treatments to the same extend. The opposite was observed here (Fig. 2). Furthermore, while targeted nitrogen remineralization in a system that was high in dissolved nitrogen (Table 1) is not likely, a remineralization of nitrogen from dead particles to an extend that could yield such a C:N shift would likely have to entail a decrease of PON in the particulate organic matter, which was not observed. Relative to the initial samples and taking the dilution factor of each treatment into consideration, POC and PON increased in 32 and 25 out of 36 bottles, respectively (3 experiments times 12 bottles each, data accessible via PANGAEA). Hence, an increase of POC is the more probable reason for the C:N shift. Two likely scenarios exist for this observation, both focusing on the < 2 µm plankton community:
High C:N picoplankton growth
It is possible that the increase of the C:Nall ratio was due to the growth of very small (< 2 µm), fast reproducing cells with high C:N ratio. At all three stations, size-fractionated initial POC:PON values revealed that the C:N ratio in the < 2 µm size fraction was 20–63% higher than the C:Nall ratio of the whole community (Table 1, statistically significant only at station 26 (n = 3, p = 0.0017)). It is important to keep in mind that this community of pico-cells is still a diverse mixture of different bacteria and picoeukaryotes with variable C:N ratios. LDNA bacteria outgrew all other measured parameters at stations 26 and 106 when grazing pressure was relieved. Although bacteria are known to have low C:N ratios of 3.6–6.8 (Fagerbakke et al. 1996; Fukuda et al. 1998) the sources do not discriminate between HDNA and LDNA bacteria. According to Geider and La Roche 2002, DNA, RNA and proteins are characterized by a particularly low C:N ratio (2.6, 2.5 and 3.8 respectively) and can contribute significantly to the cells’ biomass (0.5–3%, 3–15% and 30–65%, respectively) of phytoplankton and cyanobacteria. The C:N ratio of an organism containing low ratios of these compounds would therefore be driven by compounds with a high carbon content, such as lipids, carbohydrates and phosphoglycerides and consequently exhibit a high C:N ratio. Consistent with this, the highest growth– and mortality rates measured in all three experiments were those of POC and LDNA bacteria, which by definition carry low quantities of DNA. The fast gross growth rates of LDNA bacteria were countered by almost equally high grazing rates at all stations, resulting in balanced µ values in the undiluted seawater (1.0 dilution) bottles. With increasing dilution however, the gross growth rate of every parameter remained unaltered, while the SZP grazing pressure decreased sequentially from the undiluted (1.0 dilution) to the strongly diluted (0.25 dilution) bottles. Since the community’s POC:Chl a ratio also increased with increasing dilutions at all three stations by a factor of ~ 2.1, ~ 3.1 and ~ 2.3 at stations 26, 61 and 106, respectively (comparison between the stations’ initial samples and the strongly diluted 0.25 bottles yielded increases of 26: 731 ± 30 to 1523 ± 303 µg µg−1, 61: 105 ± 6 to 326 ± 38 µg µg−1 and 106: 136 ± 9 to 317 ± 28 µg µg−1), it can be assumed that mainly heterotrophic organisms were responsible for the shift of the communities C:Nall ratio. Therefore, it is plausible that heterotrophic bacteria and potentially other groups of heterotrophic picoplankton with high growth rates and putatively very high cellular C:N ratios thrived, once grazing pressure was released, thus leading to the observed increase in C:Nall of the whole community.
Transparent exopolymer particles
Conversely, the increasing C:N ratio with increasing dilution could be explained by the production of transparent exopolymer particles (TEP). TEP can contribute to the POC concentration equally to conventional particles (Mari 1999; Engel and Passow 2001). Furthermore, TEP have a C:N of 26 (Engel and Passow 2001) which is 4.3 times higher than the expected C:N ratio of phytoplankton in eutrophic polar waters (C:N:P of 78:13:1 equals C:N of 6 (Martiny et al. 2013)). The possibly high concentration of TEP in concert with their high C:N ratio, generally enables them to influence the C:N ratio significantly, as was observed in this study with increasing dilution. Bacteria increase TEP concentrations in multiple ways, even in concert with phytoplankton. First, bacteria produce TEP themselves (Sugimoto et al. 2007). While the appearance of TEP in situ is usually best explained by the amount of Chl a (Zamanillo et al. 2019), bacteria are the second most important group to explain TEP occurrence (Corzo et al. 2005) particularly under the mixed layer (Ortega-Retuerta et al. 2009). Second bacteria have been observed to indirectly mediate TEP production on a scale that exceeded total bacterial carbon utilization by 1–twofold (Sugimoto et al. 2007). And third bacteria have been observed to induce TEP production in phytoplankton (Van Oostende et al. 2013).
Since TEP are routinely measured by filtering samples on a 0.4 µm polycarbonate filter (Passow and Alldredge 1995), a significant share of TEP would have been retained by the GF/F filters (pore size ~0.6 µm) used in this study. High gross growth rates of bacteria were observed in the experiments at all 3 stations. Therefore, it is plausible that bacteria increased POC far beyond their own incorporation of DOC into bacterial biomass by direct formation of TEP, the mediation of the TEP-precursors’ aggregation to TEP and the induction of TEP formation in the present phytoplankton. The observed strong increase of POC with increasing dilution, in comparison with the only moderate increase of PON is in line with this argumentation.
TEP themselves, which can reach up to 100 µm in size (Passow and Alldredge 1995), aggregates bound by TEP (Larsson et al. 2022) as well as their dissolved precursors can be directly utilized as a carbon source by heterotrophic grazers (Tranvik et al. 1993; Passow and Alldredge 1999). There is no reason to believe that the TEP production rate was affected by the dilution itself. Consequently, it appears plausible that TEP have characteristic features of a prey item in the context of a dilution experiment and that with decreasing grazer concentration more TEP could prevail. As a side effect, the increasing dilution thusly also increased the C:N ratio of the particulate organic matter. Furthermore, the ecological role of TEP is far more complex than serving as prey for heterotrophic organisms. TEP constitute substrate for bacteria (Corzo et al. 2005) and as a coagulation agent (Engel and Passow 2001; Larsson et al. 2022), amplify the sedimentation of particulate organic matter. If indeed TEP significantly reacted to the dilution in these experiments, the presence of SZP grazers reduced the potential for carbon sequestration far beyond the direct disintegration of living cells into DOC and DIC by a direct consumption of TEP.
Assessing the use of POC for dilution experiments—Carbon complements Chl a
Dilution studies conducted in general, and around the WAP in particular (Burkill et al. 1995; Tsuda and Kawaguchi 1997; Garzio et al. 2013), rarely reported carbon based results (Calbet and Landry 2004) and instead relied on Chl a and cell counts. However, our results underline that it is important to include POC into the set of measured parameters for several reasons: POC provided significant correlations with the dilution factor more reliably than Chl a. While Chl a only yielded interpretable results for stations 26 and 106, POC was interpretable at all three stations. POC measurements also allowed for a direct assessment of carbon dynamics, independent of Chl a/carbon conversion factors. Former studies either calculated total PP, or the percentage of PP grazed by SZP (Calbet and Landry 2004; Pearce et al. 2008) based on Chl a results. However, it is crucial to keep in mind that this Chl a derived PP only represents a share of the total POC production in the system. The size of this share may be highly variable in between stations, as shown in this study, where the gross growth rate of POC was approximately twice as high as the gross growth rate of Chl a at station 26. Furthermore, depending on the stations characteristics, the actual share of grazed carbon may not be depicted with certainty based on Chl a derived carbon values alone. As seen at station 26 the share of SZP grazing induced mortality of Chl a in the gross Chl a production (gChl a:kChl a) amounted to only 36 ± 16% (calculated from g and k numbers in Table 2). If Chl a represented the carbon dynamics accurately, the 1.0 dilution bottles should show an increase in POC after the experiment, something that was not consistent with the 16% less POC at the end of the experiment. In contrast, the share of grazed POC in the gross POC production (gPOC:kPOC), amounted to 119 ± 16% at station 26 and thus may explain the loss of POC in the 1.0 dilution bottles. This difference between measured Chl a and carbon dynamics is crucial for the determination of the POC’s fate in the context of SZP grazing. Also, it is important to note that POC included heterotrophic organisms, not just Chl a containing phototrophs and that especially in HNLC regions of the SO, where phytoplankton are Fe-limited, Chl a may not necessarily be a good indicator of phytoplankton biomass or changes thereof (Twining and Baines 2013). Additionally, the production and consumption of TEP would not be included if only Chl a based growth rates were used.
As a general remark, the results suggested that 14C PP measurements should be interpreted cautiously in the context of dilution experiments, since the 14C PP measurements in this study showed increasing gaps to the gross absolute POC production rate (µabs POC) with increasing grazing intensity. At station 61, PP (Table 1) accounted for ~67% of the µabs POC (Fig. 4b), at station 106 only for ~ 28% of the µabs POC (763 ± 745 mg C m−2 d−1) and at station 26, the PP (Table 1) was positive, while the µabs POC (Fig. 4a) was negative. Since phytoplankton and SZP are inseparable by filtration, phytoplankton growth and SZP grazing occur simultaneously in the bottles of the 14C assay, with the latter possibly affecting the former (Moigis 1999; Marra et al. 2012). 14C is fixed, grazed, incorporated into SZP biomass, respired into the DIC pool and egested into the DOC pool all at the same time. While some studies dismiss grazing as a significant factor for 14C uptake assays (Calbet and Landry 2004; Mosby and Smith 2015), published discussion on the topic and our results, suggest that respiration and DOC excretion may constitute a significant sink of particulate 14C, especially when the grazing activity is high, potentially decreasing the measured PP. Together, our results suggest that the addition of POC to the set of measured parameters in dilution experiments leads to much better interpretation of SZP grazing activity.
SZP grazing recycled 20–50 times more carbon than was exported to depth
Upon relieve of grazing pressure, the observed increase of the C:Nall ratios in the plankton communities at all three stations (Fig. 2) suggested that SZP might either prey on cells with a comparatively high POC content, or on TEP, which have a high C:N ratio, indicated by the 2.5 times higher gPOC in comparison to the gPON (g values from station 26). However, whether this was a result of the SZP targeting these high carbon, low nitrogen particles, or an effect of cells with a low C:N ratio being better protected against grazing remains unresolved. Calbet & Landry (2004) postulated a 50% loss of carbon ingested by microzooplankton due to respiration. Taking this into account it can be assumed that an additional share of POC in the dilution experiments was converted to DOC and excreted. In addition to the carbon released from particles egested by the microplankton, organisms with a low C:N ratio may have been avoided, leading to species with predominantly low C:N being transferred to higher grazers, which then form fecal pellets which facilitate carbon export. Thus via these two processes, SZP may attenuate or even impede carbon export to depth. Thusly, the higher the SZP’s consumption of POC production, the lower the potential carbon export to depth.
Comparing all 3 stations investigated during this study, the absolute SZP grazing on POC calculated from Eq. 3 was highest at station 26 and lowest at 61. Figure 4 shows a comparison of these two contrasting stations, assuming a relatively well-mixed upper water column to a depth of 100 m. The observations made at the two stations are not intended to represent general regional characteristic features, but attempt to highlight the variable influence SZP grazing can have on the plankton community in the SO. At station 26, 119% (3567 ± 290 mg C m−2 d−1, Eq. 3) of the gross absolute POC produced was eliminated from the POC pool by SZP grazing (Fig. 4a) leaving no POC production for export to depth and resulting in a turnover time of 1.8 ± 0.2 days for the total POC pool (Table 2). In contrast, at station 61 only 56% (1565 ± 310 mg C m−2 d−1) of the gross POC production (Fig. 4b) was eliminated from the particulate pool by SZP grazing. This value represents the middle of a range of grazing values calculated from Chl a, by Burkill et al. 1995. Consequently, 44% (1230 ± 850 mg C m−2 d−1) of gross absolute POC produced was left for net POC production. Pauli et al. 2021a, b reported a particulate carbon flux of 69 ± 24 mg C m−2 d−1 (Pauli et al. 2021a) at a depth of 100 m during PS112 off Elephant Island, that predominantly consisted of krill and salp fecal pellets, meaning only < 3% of produced POC at station 61 was exported. Furthermore, 20 times more POC was eliminated from the particulate pool by SZP grazing than by carbon export at station 61 and at station 26, 50 times more POC was eliminated by SZP grazing than was exported at station 61. The remaining 41% of gross absolute POC production at station 61 must therefore have been retained in the upper 100 m, possibly due to the activity of mesozooplankton grazers (Iversen et al. 2017) or as an addition to the standing stock particulate carbon pool.
In line with previous studies, our work demonstrates that SZP plays a dominant role in the carbon cycling of the upper SO (Tsuda and Kawaguchi 1997; Schmoker et al. 2013). Interestingly, across all 3 stations, SZP grazing was further evidenced by the short residence times of POC, which ranged between 2 and 3 days (Table 2). Since the residence times of LDNA bacteria at stations 26 and 106, were ~7.7 and ~4.1 times lower than the residence times of Chl a, respectively (Table 2), it is likely that a significant portion of POC recycling encompassed the growth and consumption of small heterotrophic organisms in contrast to Chl a containing autotrophs. Furthermore, the significance of SZP in POC recycling raises the question, if their grazing activity might also significantly contribute to the recycling of other potentially limiting nutrients and trace elements like iron and manganese (Balaguer et al. 2022) or vitamins (Koch et al. 2011), as suggested in the literature (Sarthou et al. 2008).
Possible factors influencing microzooplankton grazing induced mortality
In line with previous studies (Garrison 1991; Garzio et al. 2013), which assessed the role of microzooplankton in the SO, SZP abundance and grazing induced mortality rates on the plankton community differed at the three stations. This can be explained by the following aspects:
Top down: microzooplankton abundance
During this study, heterotrophic microplankton (including heterotrophic dinoflagellates, tintinnids, aloricate ciliates and micrometazoans) showed a heterogeneous distribution, varying greatly in abundance in the South Shetland area (3–84 individuals L−1) and around Elephant island (4–81 individuals L−1), areas close to all stations in this study (Monti-Birkenmeier et al. 2021). Although not measured it is plausible, that the density and activity of higher consumers capable of feeding on microzooplankton influenced their abundance. The mesozooplankton community composition also differed greatly between the chosen experimental stations (26: 889 individuals m−3, 106: 68 individuals m−3) and over time (61: 496 and 2539 individuals m−3 within a sampling period of 18 days) (Plum et al. 2021). Salps (von Harbou et al. 2011; Pauli et al. 2021b) and Krill (Wickham and Berninger 2007; Siegel 2016; Pauli et al. 2021b) graze on members of the micro- and mesozooplankton and occur as dense swarms capable of locally grazing down the phytoplankton community with high efficiency, outcompeting other zooplankton (Perissinotto and Pakhomov 1998; Siegel 2016). However, during the PS112 expedition, both grazers had a patchy distribution (Pauli et al. 2021a), with salps ranging from 3 to 4750 ind. 1000 m−3, 47–2707 ind. 1000 m−3 and 0–251 ind. 1000 m−3 close to stations 26, 61 and 106, respectively (R. Driscoll, Bremerhaven, personal communication). In comparison, adult krill was much less abundant, but also patchy, with densities of 0–8 ind. 1000 m−3, 0–147 ind. 1000 m−3 and 0–112 ind. 1000 m−3 at stations 26, 61 and 106, respectively (R. Driscoll, Bremerhaven, personal communication).
Hence, the group-specific influences that different grazers imposed on the system at the time it was sampled were likely very different, even within a comparably small area. Therefore, the presence of higher consumers are one aspect that may explain the patchiness of SZP grazing rates, illustrated by the differences between the investigated stations. The focus of historical studies on mesozooplankton grazing combined with a lack of studies examining SZP activity have resulted in a general disregard of the importance of the microbial loop in the tertiary productivity of Antarctic ecosystems, many of which suffer from low primary productivity compared to lower latitude systems (Azam et al. 1991).
Bottom up: nutrient supply
While the dilution series method is based on the premise that only the encounter rate of predator and prey are reduced while the water chemistry remains the same, it does result in reduced demand for resources in the highly diluted treatments compared to the undiluted control. Some dilution studies thus add dissolved inorganic macronutrients to the incubations (nitrate, phosphate, silicate), in order to prevent nutrient limitation from reducing the apparent growth rates (r) of the undiluted treatments. Nutrient limitation of the undiluted bottles could increase the slope of the regression, thus overestimating the SZP grazing induced mortality (g). This was not done in this study for three main reasons: (i) Macronutrient amendments are necessary in coastal regions, which harbor high biomass and where macronutrients, especially dissolved inorganic nitrogen, often limit phytoplankton production. In contrast, in the SO, where macronutrients are generally high and biomass is low (Table 1) those amendments are not necessary. (ii) 11 of the published 16 dilution studies performed in the Southern Ocean (Burkill et al. 1995; Froneman and Perissinotto 1996a, b; Tsuda and Kawaguchi 1997; Li et al. 2001; Froneman 2004; Safi et al. 2007; Pearce et al. 2008, 2010; Mosby and Smith 2015; Christaki et al. 2021) did not add nutrients. (iii) The original method instructs that it is important to not artificially increase growth factors during the incubation (Landry and Hassett 1982). Therefore, no trace metals were added since iron limitation at these SO ocean sites was expected. An addition of iron in this context would have entailed the risk to fertilize the experimental bottles, and thereby deviate from the ambient conditions in the ocean, resulting in artificially increased r and significantly underestimated g values. The fact that the measured g values in this study even fall in the lower range of values published in the literature for the SO (Table 2 in Garzio et al. 2013), validates the decision not to add iron. Some studies have suggested that heterotrophic bacteria in the SO may be limited by the availability of carbon, rather than iron (Fourquez et al. 2020). Since DOC was not monitored during the experiments, we cannot rule out that DOC limitation may have acted together with SZP grazing to influence the mortality rates of bacteria (gbacteria all) in the undiluted bottles. However, it is worth noting that in those bottles more DOC was likely produced by higher absolute grazing intensity (Pearce et al. 2010) and that the gbacteria all in this study fell well into the range of bacterial g values published in the literature for the Southern Ocean (Table 3 in Garzio et al. 2013) strengthening the validity of the measured values of this study.
Conclusion
Our study highlights the regionally variable influence of SZP grazing on carbon cycling, by characterizing 3 very different systems. The study suggests that POC can be a valuable addition to Chl a measurements in the context of dilution experiments. Relatively short geographic distances between stations were enough to alter the influence of SZP grazing dramatically, likely due to the patchy distribution of SZP organisms and higher grazers. However, the strong overall contribution of SZP grazing to the loss of gross absolute POC production (56%-119%) suggests that SZP plays an important role in the carbon cycling at these SO sites. Furthermore, it was found that in the absence of SZP grazing, community C:N ratio rose significantly. Remineralization of carbon in the top 100 m was responsible for 20–50 times more POC recycling than was exported to depth. Thus, SZP grazing is an important removal factor to consider in global carbon cycle models since it may drastically impede the amount of carbon being exported to depth. The rates presented here fall within the range of the previously published 0.03–0.52 d−1 for the SO and corroborates previously published work, which showed that SZP grazing is highly variable in the SO in general (Schmoker et al. 2013) and around the WAP in particular. As a result, the WAP region is characterized by a high degree of patchiness and adaptability to short-term change (Tsuda and Kawaguchi 1997), such as phytoplankton bloom events due to episodic iron inputs. To investigate the existence and effects of reoccurring rhythms in this system, far more research is necessary than has been so far directed towards the climatically and ecologically pivotal system that is the WAP.
References
Agis M, Granda A, Dolan JR (2007) A cautionary note: examples of possible microbial community dynamics in dilution grazing experiments. J Exp Mar Bio Ecol 341:176–183. https://doi.org/10.1016/j.jembe.2006.09.002
Altman N, Krzywinski M (2015) Simple linear regression. Nat Methods 12:999–1000. https://doi.org/10.1038/nmeth.3627
Atkinson A, Siegel V, Pakhomov E, Rothery P (2004) Long-term decline in krill stock and increase in salps within the Southern Ocean. Nature 432:100–103. https://doi.org/10.1038/nature02996
Atkinson A, Hill SL, Pakhomov EA et al (2019) Krill (Euphausia superba) distribution contracts southward during rapid regional warming. Nat Clim Chang 9:142–147. https://doi.org/10.1038/s41558-018-0370-z
Azam F, Fenchel T, Field J et al (1983) The ecological role of water-column microbes in the sea. Mar Ecol Prog Ser 10:257–263. https://doi.org/10.3354/meps010257
Azam F, Smith DC, Hollibaugh JT (1991) The role of the microbial loop in Antarctic pelagic ecosystems. Polar Res 10:239–244. https://doi.org/10.3402/polar.v10i1.6742
Balaguer J, Koch F, Hassler C, Trimborn S (2022) Iron and manganese co-limit the growth of two phytoplankton groups dominant at two locations of the Drake Passage. Commun Biol 5:1–12. https://doi.org/10.1038/s42003-022-03148-8
Berk SG, Brownlee DC, Heinle DR et al (1977) Ciliates as a food source for marine planktonic copepods. Microb Ecol 4:27–40. https://doi.org/10.1007/BF02010427
Böckmann S, Koch F, Meyer B et al (2021) Salp fecal pellets release more bioavailable iron to Southern Ocean phytoplankton than krill fecal pellets. Curr Biol 31:1–10. https://doi.org/10.1016/j.cub.2021.02.033
Boyd PW (2002) Environmental factors controlling phytoplankton processes in the Southern Ocean. J Phycol 38:844–861. https://doi.org/10.1046/j.1529-8817.2002.t01-1-01203.x
Burkill PH, Edwards ES, Sleight MA (1995) Microzooplankton and their role in controlling phytoplankton growth in the marginal ice zone of the Bellingshausen Sea. Deep Sea Res Part II Top Stud Oceanogr 42:1277–1290. https://doi.org/10.1016/0967-0645(95)00060-4
Cabanes DJE, Blanco-Ameijeiras S, Bergin K et al (2020) Using Fe chemistry to predict Fe uptake rates for natural plankton assemblages from the Southern Ocean. Mar Chem 225:103853. https://doi.org/10.1016/j.marchem.2020.103853
Calbet A, Landry MR (2004) Phytoplankton growth, microzooplankton grazing, and carbon cycling in marine systems. Limnol Oceanogr 49:51–57. https://doi.org/10.4319/lo.2004.49.1.0051
Calbet A, Saiz E (2005) The ciliate-copepod link in marine ecosystems. Aquat Microb Ecol 38:157–167. https://doi.org/10.3354/ame038157
Calbet A, Saiz E (2013) Effects of trophic cascades in dilution grazing experiments: from artificial saturated feeding responses to positive slopes. J Plankton Res 35:1183–1191. https://doi.org/10.1093/plankt/fbt067
Calbet A, Saiz E, Almeda R et al (2011) Low microzooplankton grazing rates in the Arctic Ocean during a Phaeocystis pouchetii bloom (Summer 2007): fact or artifact of the dilution technique? J Plankton Res 33:687–701. https://doi.org/10.1093/plankt/fbq142
Calbet A, Martínez RA, Isari S et al (2012) Effects of light availability on mixotrophy and microzooplankton grazing in an oligotrophic plankton food web: evidences from a mesocosm study in Eastern Mediterranean waters. J Exp Mar Bio Ecol 424–425:66–77. https://doi.org/10.1016/j.jembe.2012.05.005
Caron DA, Dennett MR, Lonsdale DJ et al (2000) Microzooplankton herbivory in the Ross Sea, Antarctica. Deep Sea Res Part II Top Stud Oceanogr 47:3249–3272. https://doi.org/10.1016/S0967-0645(00)00067-9
Christaki U, Skouroliakou ID, Delegrange A et al (2021) Microzooplankton diversity and potential role in carbon cycling of contrasting Southern Ocean productivity regimes. J Mar Syst. https://doi.org/10.1016/j.jmarsys.2021.103531
Corzo A, Rodríguez-Gálvez S, Lubian L et al (2005) Spatial distribution of transparent exopolymer particles in the Bransfield Strait, Antarctica. J Plankton Res 27:635–646. https://doi.org/10.1093/plankt/fbi038
Ducklow HW, Baker K, Martinson DG et al (2007) Marine pelagic ecosystems: the West Antarctic Peninsula. Philos Trans R Soc B Biol Sci 362:67–94. https://doi.org/10.1098/rstb.2006.1955
Dussart BH (1965) Les différentes catégories de plancton. Hydrobiologia 26:72–74. https://doi.org/10.1007/BF00142255
Edler L (1979) Recommendations on methods for marine biological studies in the Baltic Sea. Phytoplankton and chlorophyll. Balt Mar Biol Publ 5:1–38
Engel A, Passow U (2001) Carbon and nitrogen content of transparent exopolymer particles (TEP) in relation to their Alcian Blue adsorption. Mar Ecol Prog Ser 219:1–10. https://doi.org/10.3354/meps219001
Fagerbakke K, Heldal M, Norland S (1996) Content of carbon, nitrogen, oxygen, sulfur and phosphorus in native aquatic and cultured bacteria. Aquat Microb Ecol 10:15–27. https://doi.org/10.3354/ame010015
Field CB, Behrenfeld MJ, Randerson JT, Falkowski P (1998) Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281:237–240. https://doi.org/10.1126/science.281.5374.237
Fourquez M, Bressac M, Deppeler SL et al (2020) Microbial competition in the subpolar Southern Ocean: an Fe–C Co-limitation experiment. Front Mar Sci 6:1–15. https://doi.org/10.3389/fmars.2019.00776
Froneman PW (2004) Protozooplankton community structure and grazing impact in the eastern Atlantic sector of the Southern Ocean in austral summer 1998. Deep Res Part II Top Stud Oceanogr 51:2633–2643. https://doi.org/10.1016/j.dsr2.2004.09.001
Froneman P, Perissinotto R (1996a) Microzooplankton grazing in the Southern Ocean: implications for the carbon cycle. Mar Ecol 17:99–115. https://doi.org/10.1111/j.1439-0485.1996.tb00493.x
Froneman PW, Perissinotto R (1996b) Microzooplankton grazing and protozooplankton community structure in the South Atlantic and in the Atlantic sector of the Southern Ocean. Deep Res Part I Oceanogr Res Pap 43:703–721. https://doi.org/10.1016/0967-0637(96)00010-6
Fukuda R, Ogawa H, Nagata T, Koike I (1998) Direct determination of carbon and nitrogen contents of natural bacterial assemblages in marine environments. Appl Environ Microbiol 64:3352–3358. https://doi.org/10.1128/AEM.64.9.3352-3358.1998
Garrison DL (1991) An overview of the abundance and role of protozooplankton in Antarctic waters. J Mar Syst 2:317–331. https://doi.org/10.1016/0924-7963(91)90039-W
Garzio L, Steinberg D, Erickson M, Ducklow H (2013) Microzooplankton grazing along the Western Antarctic Peninsula. Aquat Microb Ecol 70:215–232. https://doi.org/10.3354/ame01655
Gasol JM, Zweifel UL, Peters F et al (1999) Significance of size and nucleic acid content heterogeneity as measured by flow cytometry in natural planktonic bacteria. Appl Environ Microbiol 65:4475–4483. https://doi.org/10.1128/AEM.65.10.4475-4483.1999
Geider R, La Roche J (2002) Redfield revisited: variability of C:N:P in marine microalgae and its biochemical basis. Eur J Phycol 37:1–17. https://doi.org/10.1017/S0967026201003456
Grasshoff K, Ehrhardt M, Kremling K (eds) (1999) Methods of seawater analysis. Wiley-VCH Verlag GmbH, Weinheim
Henson SA, Sanders R, Madsen E (2012) Global patterns in efficiency of particulate organic carbon export and transfer to the deep ocean. Global Biogeochem Cycles. https://doi.org/10.1029/2011GB004099
Holmes RM, Aminot A, Kérouel R et al (1999) A simple and precise method for measuring ammonium in marine and freshwater ecosystems. Can J Fish Aquat Sci 56:1801–1808. https://doi.org/10.1139/f99-128
Iversen MH, Pakhomov EA, Hunt BPV et al (2017) Sinkers or floaters? Contribution from salp pellets to the export flux during a large bloom event in the Southern Ocean. Deep Sea Res Part II Top Stud Oceanogr 138:116–125. https://doi.org/10.1016/j.dsr2.2016.12.004
Koch F, Marcoval MA, Panzeca C et al (2011) The effect of vitamin B12 on phytoplankton growth and community structure in the Gulf of Alaska. Limnol Oceanogr 56:1023–1034. https://doi.org/10.4319/lo.2011.56.3.1023
Landry MR, Hassett RP (1982) Estimating the grazing impact of marine micro-zooplankton. Mar Biol 67:283–288. https://doi.org/10.1007/BF00397668
Larsson ME, Bramucci AR, Collins S et al (2022) Mucospheres produced by a mixotrophic protist impact ocean carbon cycling. Nat Commun 13:1–15. https://doi.org/10.1038/s41467-022-28867-8
Li C, Sun S, Zhang G, Ji P (2001) Summer feeding activities of zooplankton in Prydz Bay, Antarctica. Polar Biol 24:892–900. https://doi.org/10.1007/s003000100292
Löder MGJ, Meunier C, Wiltshire KH et al (2011) The role of ciliates, heterotrophic dinoflagellates and copepods in structuring spring plankton communities at Helgoland Roads, North Sea. Mar Biol 158:1551–1580. https://doi.org/10.1007/s00227-011-1670-2
Mari X (1999) Carbon content and C: N ratio of transparent exopolymeric particles (TEP) produced by bubbling exudates of diatoms. Mar Ecol Prog Ser 183:59–71. https://doi.org/10.3354/meps183059
Marra J, Capuzzo E, Montecino V (2012) Potential grazing effects in incubations with 14C. Aquat Biol 14:283–288. https://doi.org/10.3354/ab00403
Martin JH, Fitzwater SE, Gordon RM (1990) Iron deficiency limits phytoplankton growth in Antarctic waters. Global Biogeochem Cycles 4:5–12. https://doi.org/10.1029/GB004i001p00005
Martiny AC, Pham CTA, Primeau FW et al (2013) Strong latitudinal patterns in the elemental ratios of marine plankton and organic matter. Nat Geosci 6:279–283. https://doi.org/10.1038/ngeo1757
Martiny AC, Vrugt JA, Lomas MW (2014) Concentrations and ratios of particulate organic carbon, nitrogen, and phosphorus in the global ocean. Sci Data 1:140048. https://doi.org/10.1038/sdata.2014.48
McNair HM, Morison F, Graff JR et al (2021) Microzooplankton grazing constrains pathways of carbon export in the subarctic North Pacific. Limnol Oceanogr 66:2697–2711. https://doi.org/10.1002/lno.11783
Meredith MP, Stefels J, van Leeuwe M (2017) Marine studies at the western Antarctic Peninsula: priorities, progress and prognosis. Deep Sea Res Part II Top Stud Oceanogr 139:1–8. https://doi.org/10.1016/j.dsr2.2017.02.002
Moigis AG (1999) Photosynthetic rates in the surface waters of the Red Sea: the radiocarbon versus the non-isotopic dilution method. J Plankton Res 22:713–727. https://doi.org/10.1093/plankt/22.4.713
Monti-Birkenmeier M, Diociaiuti T, Badewien TH et al (2021) Spatial distribution of microzooplankton in different areas of the northern Antarctic Peninsula region, with an emphasis on tintinnids. Polar Biol 44:1749–1764. https://doi.org/10.1007/s00300-021-02910-8
Mosby AF, Smith WO (2015) Phytoplankton growth rates in the Ross Sea, Antarctica. Aquat Microb Ecol 74:157–171. https://doi.org/10.3354/ame01733
Orchard MJ, Humphries S, Schuech R, Menden-Deuer S (2016) The influence of viscosity on the motility and sensory ability of the dinoflagellate Heterocapsa triquetra. J Plankton Res 38:1062–1076. https://doi.org/10.1093/plankt/fbw004
Ortega-Retuerta E, Reche I, Pulido-Villena E et al (2009) Uncoupled distributions of transparent exopolymer particles (TEP) and dissolved carbohydrates in the Southern Ocean. Mar Chem 115:59–65. https://doi.org/10.1016/j.marchem.2009.06.004
Paffenhöfer G-A (1998) Heterotrophic protozoa and small metazoa: feeding rates and prey-consumer interactions. J Plankton Res 20:121–133. https://doi.org/10.1093/plankt/20.1.121
Passow U, Alldredge AL (1995) A dye-binding assay for the spectrophotometric measurement of transparent exopolymer particles (TEP). Limnol Oceanogr 40:1326–1335. https://doi.org/10.4319/lo.1995.40.7.1326
Passow U, Alldredge AL (1999) Do transparent exopolymer particles (TEP) inhibit grazing by the euphausiid Euphausia pacifica? J Plankton Res 21:2203–2217. https://doi.org/10.1093/plankt/21.11.2203
Pauli N-C, Flintrop CM, Konrad C et al (2021a) Krill and salp faecal pellets contribute equally to the carbon flux at the Antarctic Peninsula. Nat Commun 12:7168. https://doi.org/10.1038/s41467-021-27436-9
Pauli N-C, Metfies K, Pakhomov EA et al (2021b) Selective feeding in Southern Ocean key grazers—diet composition of krill and salps. Commun Biol 4:1061. https://doi.org/10.1038/s42003-021-02581-5
Pearce I, Davidson AT, Wright S, Van Den Enden R (2008) Seasonal changes in phytoplankton growth and microzooplankton grazing at an Antarctic coastal site. Aquat Microb Ecol 50:157–167. https://doi.org/10.3354/ame01149
Pearce I, Davidson AT, Thomson PG et al (2010) Marine microbial ecology off East Antarctica (30–80°E): rates of bacterial and phytoplankton growth and grazing by heterotrophic protists. Deep Res Part II Top Stud Oceanogr 57:849–862. https://doi.org/10.1016/j.dsr2.2008.04.039
Perissinotto RA, Pakhomov E (1998) The trophic role of the tunicate Salpa thompsoni in the Antarctic marine ecosystem. J Mar Syst 17:361–374. https://doi.org/10.1016/S0924-7963(98)00049-9
Plum C, Cornils A, Driscoll R et al (2021) Mesozooplankton trait distribution in relation to environmental conditions and the presence of krill and salps along the northern Antarctic Peninsula. J Plankton Res 43:927–944. https://doi.org/10.1093/plankt/fbab068
Price LM (2012) Microzooplankton Community Structure and Grazing Impact Along the Western Antarctic Peninsula. College of William and Mary
Redfield AC, Ketchum BH, Richards FA (1963) The influence of organisms on the composition of sea-water. In: Hill MN (ed) In The Sea. Ideas and observations on progress in the study of the sea. Wiley Interscience, New York, pp 26–77
Ryther JH (1969) Photosynthesis and fish production in the sea. Science 166:72–76. https://doi.org/10.1126/science.166.3901.72
Safi KA, Brian Griffiths F, Hall JA (2007) Microzooplankton composition, biomass and grazing rates along the WOCE SR3 line between Tasmania and Antarctica. Deep Res Part I Oceanogr Res Pap 54:1025–1041. https://doi.org/10.1016/j.dsr.2007.05.003
Sarthou G, Vincent D, Christaki U et al (2008) The fate of biogenic iron during a phytoplankton bloom induced by natural fertilisation: impact of copepod grazing. Deep Res Part II Top Stud Oceanogr 55:734–751. https://doi.org/10.1016/j.dsr2.2007.12.033
Schmidt K, Atkinson A, Petzke K-J et al (2006) Protozoans as a food source for Antarctic krill, Euphausia superba: complementary insights from stomach content, fatty acids, and stable isotopes. Limnol Oceanogr 51:2409–2427. https://doi.org/10.4319/lo.2006.51.5.2409
Schmoker C, Hernández-León S, Calbet A (2013) Microzooplankton grazing in the oceans: impacts, data variability, knowledge gaps and future directions. J Plankton Res 35:691–706. https://doi.org/10.1093/plankt/fbt023
Sherr EB, Sherr BF (2002) Significance of predation by protists in aquatic microbial food webs. Antonie Van Leeuwenhoek 81:293–308. https://doi.org/10.1023/A:1020591307260
Siegel V (2016) Biology and Ecology of Antarctic Krill, vol 1. Springer International Publishing, Berlin
Steinberg DK, Landry MR (2017) Zooplankton and the Ocean carbon cycle. Ann Rev Mar Sci 9:413–444. https://doi.org/10.1146/annurev-marine-010814-015924
Steinberg D, Martinson D, Costa D (2012) Two decades of pelagic ecology of the Western Antarctic Peninsula. Oceanography 25:56–67. https://doi.org/10.5670/oceanog.2012.75
Sugimoto K, Fukuda H, Baki MA, Koike I (2007) Bacterial contributions to formation of transparent exopolymer particles (TEP) and seasonal trends in coastal waters of Sagami Bay, Japan. Aquat Microb Ecol 46:31–41. https://doi.org/10.3354/ame046031
Teixeira IG, Figueiras FG (2009) Feeding behaviour and non-linear responses in dilution experiments in a coastal upwelling system. Aquat Microb Ecol 55:53–63. https://doi.org/10.3354/ame01281
Tranvik LJ, Sherr EB, Sherr BF (1993) Uptake and utilization of “colloidal DOM’’ by heterotrophic flagellates in seawater”. Mar Ecol Prog Ser 92:301–309. https://doi.org/10.3354/meps092301
Tsuda A, Kawaguchi S (1997) Microzooplankton grazing in the surface water of the Southern Ocean during an austral summer. Polar Biol 18:240–245. https://doi.org/10.1007/s003000050184
Turner JT (2015) Zooplankton fecal pellets, marine snow, phytodetritus and the ocean’s biological pump. Prog Oceanogr 130:205–248. https://doi.org/10.1016/j.pocean.2014.08.005
Twining BS, Baines SB (2013) The trace metal composition of marine phytoplankton. Ann Rev Mar Sci 5:191–215. https://doi.org/10.1146/annurev-marine-121211-172322
UNESCO (1994) Ocean Flux Study (Jgofs) Protocols for the Joint Global Core Measurements. 181
Utermöhl H (1958) Zur Vervollkommnung der quantitativen Phytoplankton Methodik. Mitteilung Int Vereinigung Für Theor Und Angew Limnol 9:1–39
Van Oostende N, Moerdijk-Poortvliet TCW, Boschker HTS et al (2013) Release of dissolved carbohydrates by Emiliania huxleyi and formation of transparent exopolymer particles depend on algal life cycle and bacterial activity. Environ Microbiol 15:1514–1531. https://doi.org/10.1111/j.1462-2920.2012.02873.x
Vaughan DG, Marshall GJ, Connolley WM et al (2003) Recent rapid regional warming on the Antarctic Peninsula. Clim Chang 60:243–274. https://doi.org/10.1023/A:1026021217991
von Harbou L, Dubischar CD, Pakhomov EA et al (2011) Salps in the Lazarev Sea, Southern Ocean: I. Feeding Dynamics Mar Biol 158:2009–2026. https://doi.org/10.1007/s00227-011-1709-4
Welschmeyer NA (1994) Fluorometric analysis of chlorophyll a in the presence of chlorophyll b and pheopigments. Limnol Oceanogr 39:1985–1992. https://doi.org/10.4319/lo.1994.39.8.1985
Wickham SA, Berninger UG (2007) Krill larvae, copepods and the microbial food web: interactions during the Antarctic fall. Aquat Microb Ecol 46:1–13. https://doi.org/10.3354/ame046001
Zamanillo M, Ortega-Retuerta E, Nunes S et al (2019) Distribution of transparent exopolymer particles (TEP) in distinct regions of the Southern Ocean. Sci Total Environ 691:736–748. https://doi.org/10.1016/j.scitotenv.2019.06.524
Acknowledgements
This study contributed to the project ‘‘Population Shift and Ecosystem Response – Krill vs. Salps’’, which was funded by the Ministry for Science and Culture of Lower Saxony (Germany). We thank Ryan Driscoll for data on krill and salp abundances in the study area. Furthermore, we thank Franziska Pausch, Anna Pagnone, Dorothee Wilhelms-Dick, and the crew of RV Polarstern for assistance during the sampling as well as Christine Klaas for constructive discussions.
Funding
Open Access funding enabled and organized by Projekt DEAL. The full original dataset can be found on PANGAEA [Böckmann, Sebastian; Trimborn, Scarlett; Koch, Florian (2024): Grazing of nano- and microzooplankton on, and growth of picoplankton and nanoplankton at Western Antarctic Peninsula, Southern Ocean. PANGAEA, https://doi.org/10.1594/PANGAEA.965603]. This work was supported by the German Research Foundation priority program SPP1158 [KO 5563/11 to F.K.]. The PhD position of S.B. in the project ‘‘Population Shift and Ecosystem Response – Krill vs. Salps,’’ [ZN 3204, Suptopic 2 ‘The impact of krill and salps on Fe biogeochemistry in Fe-limited oceanic waters’ to S.T.) was funded by the Ministry for Science and Culture of Lower Saxony (Germany).
Author information
Authors and Affiliations
Contributions
FK conceived and designed research. FK conducted the field work. SB and FK analysed the data. All authors contributed to writing, review and editing. Funding was acquired by ST and FK This study was supervized by FK and ST.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article..
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Böckmann, S., Trimborn, S., Schubert, H. et al. Grazing by nano- and microzooplankton on heterotrophic picoplankton dominates the biological carbon cycling around the Western Antarctic Peninsula. Polar Biol 47, 279–294 (2024). https://doi.org/10.1007/s00300-024-03231-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-024-03231-2