Abstract
Attaching cameras to marine mammals allows for first-hand observation of underwater behaviours that may otherwise go unseen. While studying the foraging behaviour of 26 lactating Weddell seals (Leptonychotes weddellii) in Erebus Bay during the austral spring of 2018 and 2019, we witnessed three adults and one pup investigating the cavities of Rossellidae glass sponges, with one seal visibly chewing when she removed her head from the sponge. To our knowledge, this is the first report of such behaviour. While the prey item was not identifiable, some Trematomus fish (a known Weddell seal prey) use glass sponges for shelter and in which to lay their eggs. Three of the four sponge foraging observations occurred around 13:00 (NZDT). Two of the three sponge foraging adults had higher-than-average reproductive rates, and the greatest number of previous pups of any seal in our study population, each having ten pups in 12 years. This is far higher than the study population average of three previous pups (± 2.6 SD). This novel foraging strategy may have evolved in response to changes in prey availability, and could offer an evolutionary advantage to some individuals that exploit prey resources that others may not. Our observations offer new insight into the foraging behaviours of one of the world’s most studied marine mammals. Further research on the social aspects of Weddell seal behaviour may increase our understanding of the extent and mechanisms of behavioural transfer between conspecifics. Research into the specific foraging behaviour of especially successful or experienced breeders is also warranted.
Similar content being viewed by others
Introduction
Studying the foraging of marine mammals is inherently difficult as much of it occurs underwater (Wilmers et al. 2015). The development of animal-borne video recorders (ABVRs) has made the observation of the foraging behaviours of diving animals possible (Davis et al. 1992; Marshall 1998; Moll et al. 2007). ABVRs have provided important insight into the hidden behaviours of marine fauna, and have been deployed on a variety of species, from sea turtles (Chelonioidea, Hounslow et al. 2021), tiger sharks (Galeocerdo cuvier, Heithaus et al. 2002), and Adélie penguins (Pygoscelis adeliae, Thiebot et al. 2016), to northern elephant seals (Mirounga angustirostris, Yoshino et al. 2020, Adachi et al. 2021), humpback (Megaptera novaeangliae, Akiyama et al. 2019, Iwata et al. 2021) and blue whales (Balaenoptera musculus, Calambokidis et al. 2008).
One of the first deployments of an ABVR in Antarctica reported the first observations of Weddell seals (Leptonychotes weddellii) blowing bubbles underneath the ice to flush out Trematomus borchgrevinki from the platelet ice (Davis et al. 1999). ABVRs and still cameras have since been deployed on Weddell seals on numerous occasions. Observations have included interaction between mother–pup pairs (Sato et al. 2003), the distinction between dive types (Davis et al. 2003, 2013; Fuiman et al. 2007; Madden et al. 2008), prey abundance and distribution (Watanabe et al. 2003; Mori et al. 2005), habitat use by invertebrate fauna (Watanabe et al. 2006), prey acquisition tactics (Davis et al. 1999; Sato et al. 2002), and prey species targeted (Davis et al. 1999, 2004; Fuiman et al. 2002; Foster-Dyer et al. in review).
Understanding the foraging ecology of a species is critical to understanding its role in an ecosystem and the possible challenges it may face. Weddell seals are generalist predators, known to feed on a range of prey including fishes, cephalopods, and crustaceans (Burns et al. 1998; Goetz et al. 2017). Notably, during the pup-rearing period, female Weddell seals are central place foragers, bound to forage within a limited area due to the presence of their pup (LaRue et al. 2019). Such a restriction can lead to the development of ‘foraging halos’ or areas beneath the ice with reduced prey availability (Ashmole 1963; Elliott et al. 2009). For example, Testa et al. (1985) found there was a decrease in Antarctic silverfish (Pleuragramma antarcticum) and Antarctic toothfish (Dissostichus mawsoni) in the vicinity of Weddell seal breeding colonies in Erebus Bay. Ainley et al. (2021) also suggested Weddell seal abundance may impact the toothfish scientific catch-per-unit-effort, with fewer fish being caught during Weddell seal population peaks. The prey targeted by Weddell seals also varies across location, season, and age-groups (Lindsay 1937; Burns et al. 1998; Rumolo et al. 2020), and research suggests they may show behavioural plasticity in prey selection, being able to adapt prey targeted to what is available (Foster-Dyer et al. in review).
The Weddell seal population in Erebus Bay is among the world’s most well-studied marine mammal populations, being censused every year since the late 1960s (Siniff et al. 1977; Rotella 2018). While studying the foraging behaviours of lactating Weddell seals in Erebus Bay, we opportunistically observed four seals searching for prey within the cavities of glass sponges (Rossellidae: Rossella cf. racovitzae). Here we qualitatively describe the observations and postulate hypotheses surrounding the ethological and ecological advantage conferred by the novel behaviour exhibited and suggest how and why this behaviour may have arisen.
Methods
Our methods have been outlined in detail in Foster-Dyer et al. (in review). Briefly, we instrumented 26 lactating female Weddell seals in November and December of 2018 (n = 18) and 2019 (n = 8) at six sites in Erebus Bay in the southern Ross Sea (− 77.62°: − 77.87°S, 166.3°:167.0°E, Fig. 1). Seals were immobilised using techniques described in Mellish et al. (2010), and work was covered by full ethical approval (see “Ethics approval” section for details). While anesthetised, we fitted each seal with a video camera (Little Leonardo DVL1300M130-VD3GT-2R) with red light-emitting diodes (LEDs) either on the top of the head (n = 23) or cheek (n = 3), a time-depth recorder (TDR) with acceleration and magnetism sensors (Little Leonardo ORI1300-3MPD3GT OR W1000L-3MPD3GT) to the upper back, and for the seals instrumented in 2018, an accelerometer (Little Leonardo ORI1300-D3GT OR ORI2000-D3GT) was attached under the jaw (for tag details and image of instrumented seal see Supplementary material S1–S3). Due to the limited battery life and memory capacity of the instruments, we recaptured each seal after approximately five days (mean = 4.6 ± 1.1 SD) and retrieved all equipment.
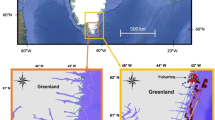
Erebus Bay, Antarctica (− 77.62°: − 77.87°S, 166.3°:167.0°E). a Map of the study area where 26 lactating female Weddell seals were fitted with time-depth recorders and animal-borne video recorders in November and December of 2018 and 2019. Red stars indicate locations at which seals were observed interacting with glass sponges. The remaining locations (black dots) are where seals were tagged, but no sponge foraging was observed. b Overall view of Ross Island in the southern Ross Sea, extent of image a indicated by red square. c Overall view of Antarctica, with extent of image b indicated by red square. Map made using Quantarctica (Matsuoka et al. 2021)
The maximum weight of the bio-logging equipment attached to each seal weighed no more than 1% of their body mass (Supplementary material S3). Due to the great depths the seals travel to, infrared (IR) light (λmax = 850 nm) is used on the video equipment to allow observations to be made in complete darkness. IR light is believed to be invisible to Weddell seals and their prey due to their short-wavelength sensitive rod opsins, which are sensitive to blue-green light (λmax = 495–499 nm; Lythgoe & Dartnall 1970; Nealson 1981; Levenson et al. 2006, Supplementary material S4).
We analysed video footage using behavioural analysis software BORIS (Friard & Gamba 2016). TDR data were analysed using diveMove (version 1.5.3; Luque 2007) in R statistical software (version 4.0.4, R Core Team 2021). We then combined the TDR-derived depth data and camera-derived prey encounter data to identify the depths at which each encounter occurred. Data on seal ages and breeding histories were provided by Drs. J. Rotella and R. Garrott (see further information under “Funding” below). The sponges investigated by the seals were identified from the videos based on their visible morphological characteristics as described by Federwisch et al. (2020).
Results
We observed a new foraging behaviour displayed by three lactating female seals and one tagged seal’s pup: searching the cavities of glass sponges (identified as Rossella cf. racovitzae, Fig. 2), presumably looking for prey. These encounters occurred at a mean depth of 154.5 m (± 56.0 SD) and were identified on six separate occasions (once by WS18-13 and WS19-39, three times by WS18-17, and once by WS18-16’s pup; summarised in Supplementary file S5, videos available in Supplementary files S6–S8). The flipper-tags of all sponge foraging adult females and their pups, and the data gathered for the study population are provided (Table 1, Supplementary files S9 and S10). Here we qualitatively describe each encounter.
Example images of glass sponges (Rossellidae: Rossella racovitzae), highlighting a sample of marine fauna that can be associated with glass sponges in Antarctica. Images were taken using ROVs in the Weddell Sea between 261 and 300 m. a Rossella racovitzae with feather stars (order Comatulida) visible on the rim and sides of the sponge, distance of lasers: 5 cm. Image copyright © Lundälv & Richter (2019), https://doi.org/10.1594/PANGAEA.897581. b Rossella racovitzae with the cavity visible at the top of the sponge and a shrimp (class Decapoda) on the rim of the sponge cavity. Image copyright © Federwisch et al. (2019), https://doi.org/10.1594/PANGAEA.897590
WS18-13 (flipper-tag #8827C)
Instrumented at Big Razorback in 2018, this unknown age seal (marked as an adult in 2007 for the first time) was accompanied by her 24-day-old pup. She had nine previously recorded pups, which when combined with information on minimal ages for first reproduction, suggests that she is at least 16 years old. The sponge foraging event occurred on 21 November 2018 at 13:07 (NZDT). The Weddell seal was on a foraging dive, swimming rapidly just above the seafloor, with the seafloor in sight. She slowed her swim speed as she came upon a large glass sponge at 239.2 m deep (Fig. 3). Several crinoids (order Comatulida) and a large starfish (class Asteroidea) were visible on the outside of the sponge. As she approached, the seal circled around the sponge before putting her head into the cavity, where it remained for six seconds. During this time, half of the video image went black due to the camera being pressed into the sponge wall. The seal then removed her head and ascended into the water column, resurfacing 10 min later. The dive lasted 20 min, and the seal reached a maximum depth of 240.7 m, with the sponge encounter occurring near the very bottom of the dive. This was the only foraging attempt observed within that dive. However, on the dive prior, the seal was observed eating three Antarctic silverfish. The sponge targeted by WS18-13 had a wide, easily accessible cavity, with the seal appearing to be able to investigate within the sponge cavity while causing little to no apparent damage to the sponge (Fig. 3, Supplementary file S6).
Images taken from video captured using an animal-borne video recorder at Big Razorback in Erebus Bay on 21 November 2018 at 13:07 (NZDT), showing Weddell seal WS18-13 presumably searching for prey within the cavity of a large glass sponge (Rossellidae: Rossella cf. racovitzae) at 239.2 m deep. The first image shows the large opening of the sponge and the second shows the seal’s head deeply within the sponge cavity. An arm of a large starfish can be seen at the top of the image (white arrows), the seal’s snout is visible at the bottom of the image (blue arrows) and the sponge cavity is indicated by the black arrows. The image appears red due to LED light on camera
WS18-16 (flipper-tag #8763C, pup flipper-tag #8370C)
On 28 November 2018 at 13:22 (NZDT), we documented this seal interacting with her 29-day-old female pup while the pup investigated a sponge cavity (Fig. 4, Supplementary file S7). WS18-16 was tagged at Big Razorback with the camera attached to her cheek rather than the top of her head. She was of unknown age but had two previous recorded pups. She was first observed as an adult with a pup in 2015 and was estimated to be at least eight years old at the time of our study. The observation occurred during an approximately 50-min period when the tagged seal and her pup had been performing repeated synchronous shallow dives (> 50 m). During this period, the adult female would depart from the breathing hole shortly after her pup and descend to the seafloor, where she would locate her pup, often nosing around the benthos. They would then interact near the seafloor for a period and swim back to the surface together.
Images taken from video captured using an animal-borne video recorder at Big Razorback on 28 November 2018 at 13:22 (NZDT), showing Weddell seal WS18-16 interacting with her 29-day old female pup while it searched within a glass sponge cavity at 47.6 m deep. Camera is attached to tagged seal’s cheek in this observation, and the side of the tagged seals face is visible in the left portion of the image, it appears red due to the LED light on camera. The large Rossella sponge can be seen in the bottom right corner (blue arrows), and the tagged seal’s pup is visible with her head inside the sponge (white arrows). The blue portion of the image is the underside of the ice above
On the final dive of this 50-min period, the tagged seal descended from the breathing hole, reaching the seafloor after approximately one minute, where she located her pup with its head inside a large Rossella sponge at 47.6 m deep. She nudged her pup once with her snout, then appeared to settle on the seafloor beside the sponge, where she remained for 10 s. The seal then continued descending with her pup, following the seafloor to a maximum depth of 77.6 m, before ascending to the surface 4.5 min after the sponge interaction. The entire dive lasted approximately 6.1 min. Due to the camera angle, the pup was not always visible. Still, she frequently appeared in the camera view, suggesting they were together for most, if not all, of the dive.
WS18-17 (flipper-tag #1043C)
This seal was tagged at North Base and her pup was 26 days old. She was of unknown age but had at least nine previous pups and was flipper-tagged as an adult with a pup in 2007 when she was observed for the first time. Given her sighting history, she was estimated to be a minimum of 16 years old. The sponge interactions occurred on 29 November 2018, beginning at 13:15 (NZDT). This seal inspected the cavities of three glass sponges (all identified as R. cf. racovitzae), travelling to each consecutively within a 2-min period at a mean depth of 159.7 m (± 4.75 SD, Fig. 5).The foraging dive lasted 16 min, reaching a maximum depth of 183.8 m.
A three-dimensional dive profile indicating the swim speed and route travelled by Weddell seal WS18-17 under the ice at North Base in Erebus Bay on 29 November 2018, beginning at 13:15 (NZDT), showing the seal searching for prey within three different glass sponges at a mean depth of 159.7 m (± 4.75 SD; green dots); inset images are taken during the sponge encounters from the video captured using a seal-mounted camera, a and b the first and second sponges investigated by the seal; c the final sponge investigated by the seal with a number of sea cucumbers (class Holothuroidea) visible around the opening on the sponge (white arrows).) The seal’s snout and fur is visible at the bottom of each image. The images appear red due to the LED light on camera
Initially, the seal descended steeply from the surface for 2.2 min, first sighting the seafloor at approximately 164 m. She followed the seafloor downward, reaching the bottom of the dive approximately 30 s later. The seal then began swimming directly above the benthos, appearing to search for prey between rocks and crevices and briefly inspecting one R. racovitzae sponge without putting her head in it. Two minutes after the reaching the bottom of the dive, the seal approached another large Rossella sponge, pressing her head through the small opening into the sponge cavity for four seconds. The seal removed her head from the sponge and continued swimming along the seafloor, encountering the next sponge 25 s later. After having her head within that sponge for seven seconds, she continued travelling along the seafloor, swimming directly above two more glass sponges without stopping.
The seal stopped at the final sponge 32 s after the previous encounter. This sponge appeared to have several sea cucumbers (class Holothuroidea) sitting at the edge of its opening, some of which were moved around by the seal. In this encounter, the seal had her head inside the sponge cavity for seven seconds. When she removed her head, she appeared to be chewing, as determined through clear head and snout movement. We could not identify what the seal was chewing as it was already inside her mouth when she removed her head from the sponge. However, she was visibly chewing for at least 15 s, suggesting the prey may have been large or difficult to swallow. After the final sponge encounter, the seal continued travelling along the seafloor for a further nine minutes before ascending to the surface. Notably, the sponge openings appeared much smaller than in the previous observation. WS18-17 appeared to forcibly press her head into a very small opening, possibly damaging the sponge (Supplementary file S8).
WS19-39 (flipper-tag #0400C)
This female was tagged at Pram Point; she had a 29-day old pup, was known age (nine years old), and had two previous pups. The sponge foraging observation occurred on 4 December 2019 at 22:10 (NZDT). The foraging dive lasted 32 min, reaching a maximum depth of 272.5 m and included many prey items, including crustaceans and Channichthyidae fish species. The seal rapidly travelled along the seafloor, after preying upon a shrimp-like crustacean, when she swam above a large R. cf. racovitzae sponge at 161.3 m. She slowed down, circled back, and nudged the sponge surface, before rapidly swimming off, possibly in pursuit of prey. However, this was unable to be determined due to image quality. The seal then ascended to the surface. This interaction was less invasive than the other observations. Still, the seal made an obvious effort to inspect the sponge and appeared to pursue prey immediately after.
Discussion
We report the first evidence of Weddell seals searching for prey within the cavities of glass sponges. Similar to the first report of Weddell seals using bubbles to remove fish from the underside of the ice (Davis et al. 1999), our sponge foraging observations highlight one of the key benefits of utilising ABVRs to observe otherwise unseen underwater behaviours displayed by this species.
Flexible and opportunistic foraging strategies, such as sponge foraging, may allow some seals to have higher reproductive rates. Two of the females observed searching sponges, WS18-13 and WS18-17, have unusually productive breeding histories. These two seals had the greatest number of pups of the seals in our study population (n = 26)—each having a minimum of nine pups since first being observed in 2007 (Table 1, Supplementary file S9). Given their breeding history, year of first observation and the average age of first reproduction for Weddell seals in Erebus Bay (− 7 years, Hadley et al. 2006), these females are at least 16 years old, making them among the oldest seals in our study population. It is possible sponge foraging arises as a function of age and experience. On average, Weddell seals that pup one year are typically less likely to pup the following year (Siniff 1981; Hadley et al. 2007). However, WS18-13 and WS18-17 each pupped in 10 out of the 12 years prior to 2019 (Supplementary file S9), giving them a higher-than-average reproductive rate for the species. While we are not suggesting looking for prey within a sponge cavity makes one a better mother, it could be that these seals show creative plasticity in their foraging tactics and that may allow them to exploit prey resources that others do not. We postulate that these individuals may display behavioural differences that allow them to be more reproductively successful than others in the population. The specific foraging behaviour of especially successful or experienced breeders is an area that would benefit from further research.
The time of day that three of the four interactions were observed, each occurring around 13:00 (NZDT), were similar. Weddell seals perform deeper dives during the day, possibly in response to the diel vertical migration patterns utilised by many of their prey (Plötz et al. 2002; Fuiman et al. 2002). When light levels are variable, Antarctic silverfish, an important prey species for Weddell seals (Burns et al. 1998), use diel vertical migration as an avoidance tactic for their visually oriented predators, such as penguins and seals (Plötz et al. 2002; Fuiman et al. 2002). Our study occurred during the late spring and summer when light levels are relatively constant; thus diel vertical migration of prey is unlikely. However, in each of the midday sponge foraging attempts, no other prey were encountered during the dives. This may be a function of prey movement through vertical migration, or possibly the ‘foraging halo’ effect discussed earlier in the text, but either way, we postulate that this novel foraging behaviour may occur when other prey are scarce. Opportunistic sponge foraging may arise independently in response to changes in prey availability and could offer an evolutionary advantage if some animals have identified the sponges as a potential location in which to find food in an otherwise sparse environment.
While it may have been merely young pup curiosity, the observation of one seal’s pup investigating a sponge raises some questions regarding how the behaviour may transfer between individuals. Social learning can occur through a number of mechanisms, such as imitation or teaching (Caro & Hauser 1992; Byrne & Russon 1998). Throughout the lactation period, Weddell seal mothers teach their offspring behaviours that will benefit them after weaning, such as diving (Sato et al. 2003). However, teaching is difficult to identify in wild animals. Caro and Hauser (1992) defined teaching as an individual modifying its behaviour only in the presence of a naïve observer, involving some cost or lack of immediate benefit to the teacher, but resulting in the observer acquiring skills earlier, or more efficiently, than it may have otherwise. Alternatively, imitation occurs when an individual incidentally learns a behaviour through observing a conspecific engaging in normal behaviour (Byrne & Russon 1998). Bandura (1989) determined that to learn a behaviour through imitation, an individual must: (1) pay attention to the behaviour of another individual, (2) remember what the modeller has done, (3) have the ability or skill to perform such behaviour, and (4) be motivated and have the opportunity to do so. Though the author’s studies focussed on learning in human children, much of it can be applied to non-human animals.
Sato et al. (2003) suggested female Weddell seals may modify their diving behaviour when diving with their pup—diving to shallower depths and swimming slower than diving alone. We suggest that some seal mothers may go further and teach their young where and how to locate and capture prey. We did not observe the tagged seal directly interacting with the sponge, nor did we observe her demonstrating the sponge foraging behaviour prior to her pup displaying it. However, as another adult displayed the sponge foraging behaviour in the same year and location (Big Razorback in 2018), albeit at depths that exceed a pup’s diving ability (Weitzner et al. 2021), it’s possible that the pup could have mimicked the behaviour after observing an adult interacting with a sponge. Our observation of the mother–pup pair diving to 77.6 m together may also provide new insight into the depths that mother–pup pairs travel to during the lactation period. Sato et al. (2003) found the maximum depth reached by a Weddell seal mother–pup pair was 51.5 m. However, Weitzner et al. (2021) reported a maximum depth reached by a Weddell seal pup to be 163.8 m, though the pup’s age, weaning status, or whether it was accompanied by its mother was not reported.
It is unclear how widespread the sponge foraging behaviour is within the Weddell seal population. We recorded sponge foraging attempts at three locations: Big Razorback, Pram Point, and North Base. Pram Point, at the southernmost region of Ross Island where Scott Base is located, is approximately 25 km south of Big Razorback Island. North Base is a further 9 km eastward at the northern edge of the Erebus Glacier Tongue (Fig. 1). Even so, opportunities exist for behaviours, such as the sponge foraging technique we report here, to be shared among conspecifics that breed at different sites within the Erebus Bay area. While Weddell seals display a high degree of site fidelity (Cameron et al. 2007), the sighting history for all four seals indicates that they previously bred at different locations across the Erebus Bay study area (Supplementary file S6). Additionally, non-breeding females are not tied to the same site they later breed at (Stirling 1974). As glass sponges, including the species R. racovitzae, have a circumpolar distribution and are found across the Ross Sea continental shelf (Janussen & Downey 2014), we speculate the sponge foraging behaviour we observed in Erebus Bay is likely displayed elsewhere.
While we lack information on the relatedness of the sponge foraging individuals, further observations of sponge foraging within the long-term Weddell seal demographic study in Erebus Bay may allow for investigation into the genetic connectivity of the behaviour. In Shark Bay, Australia, bottlenose dolphins (Tursiops sp.) have been observed “sponging”, in which dolphins carry a piece of marine sponge on their rostrum while digging through sediment looking for prey (Krützen et al. 2005, 2014). Krützen et al. (2005) found this behaviour to be culturally transmitted, usually from mother to female offspring and all adults displaying the behaviour were genetically linked to one recent sponging predecessor. The existence of culture in Weddell seals has yet to be explored. However, future observations may allow for investigation into whether the sponge foraging behaviour transfers between individuals. The sex of the pup also highlights an additional area that would benefit from further research; more data would be required to understand, (a) whether males utilise the sponge foraging technique, (b) whether mother Weddell seals pass the same traits on to their female and male offspring, and (c) the general extent of behavioural transfer between Weddell seal mothers and their pups.
Finally, a question remains regarding what the seals were targeting when searching within the sponges. The obvious chewing movements of WS18-17 (Supplementary file S8) suggest that the search can prove successful on occasion. Weddell seals are generalist predators (Burns et al. 1998; Goetz et al. 2017) and forage throughout the water column, from just below the ice surface to depths of at least 450 m (Foster-Dyer et al. in review). Marine sponges provide food, shelter, and substrate to a variety of Antarctic marine fauna (Dayton et al. 1974, Fig. 2). Although seals were only observed investigating large R. cf. racovitzae, it is unlikely the behaviour is restricted to just this sponge species. Several other Rossellidae species grow to similar or larger sizes (Barthel & Tendal 1994) and may thus likewise harbour potential prey. Various Trematomus fish species use glass sponges to shelter from predators and as nesting sites in which they lay their eggs (Dayton et al. 1974; Moreno 1980; Konecki & Targett 1989; Barthel 1997; La Mesa et al. 2019). Moreno (1980) reported that six of the seven glass sponges observed in South Bay (Doumer Island, Western Antarctic Peninsula) contained Trematomus eggs and/or adult fish. In McMurdo Sound, Dayton et al. (1974) also reported unspecified fish species using sponges to avoid predation by seals—though our observations suggest that some seals may have adapted to this predator avoidance technique.
Conclusions
We documented the first observations of Weddell seals searching for prey within the cavities of Rossellidae sponges in Erebus Bay, Antarctica. While we present the first evidence of such a behaviour, we propose that it may be displayed elsewhere around the Antarctic, given the circumpolar nature of both Rossella sponges and Weddell seals. Marine fauna use glass sponges as shelter throughout their distribution and thus may be a reliable refuge for prey. The observation of a seal’s pup investigating a sponge cavity suggests the potential for learning. The age, experience levels, and reproductive rate of WS18-13 and WS18-17 also suggest several areas of further research to better understand the relationship between foraging strategies and reproductive success. Further research into the behavioural ecology of Weddell seals and the extent of the lessons that Weddell seal pups learn from their mothers is warranted. Our findings highlight some of the key benefits of utilising animal-borne video recorders, as well as the exciting unknowns that can still be discovered for one of the world’s most well-studied marine mammals.
Data availability
All data generated or analysed for this publication are included in this published article and its supplementary information files.
References
Adachi T, Takahashi A, Costa DP, Robinson PW, Hückstädt LA, Peterson SH, Holser RR, Beltran RS, Keates TR, Naito Y (2021) Forced into an ecological corner: round-the-clock deep foraging on small prey by elephant seals. Sci Adv 7(20):eabg3628
Ainley DG, Cziko PA, Nur N, Rotella JJ, Eastman JT, Larue M, Stirling I, Abrams PA (2021) Further evidence that Antarctic toothfish are important to Weddell seals. Antarct Sci 33(1):17–29
Akiyama Y, Akamatsu T, Rasmussen MH, Iversen MR, Iwata T, Goto Y, Aoki K, Sato K (2019) Leave or stay? Video-logger revealed foraging efficiency of humpback whales under temporal change in prey density. PLoS ONE 14(2):e0211138
Ashmole NP (1963) The regulation of numbers of tropical oceanic birds. Ibis 103(3):458–473
Bandura A (1989) Human agency in social cognitive theory. Am Psychol 44(9):1175
Barthel D (1997) Fish eggs and pentacrinoids in Weddell Sea hexactinellids: further examples for the structuring role of sponges in Antarctic benthic ecosystems. Polar Biol 17(1):91–94
Barthel D, Tendal OS (1994) Antarctic Hexactinellida. In: Wägele JW, Sieg J (eds) Synopses of the Antarctic Benthos, vol 6. Koeltz Scientific Books, Koenigstein
Burns JM, Trumble SJ, Castellini MA, Testa JW (1998) The diet of Weddell seals in McMurdo Sound, Antarctica as determined from scat collections and stable isotope analysis. Polar Biol 19(4):272–282
Byrne RW, Russon AE (1998) Learning by imitation: a hierarchical approach. Behav Brain Sci 21(5):667–684
Calambokidis J, Schorr GS, Steiger GH, Francis J, Bakhtiari M, Marshall G, Oleson EM, Gendron D, Robertson K (2008) Insights into the underwater diving, feeding, and calling behavior of blue whales from a suction-cup-attached video-imaging tag (CRITTERCAM). Mar Technol Soc J 41(4):19–29
Cameron MF, Siniff DB, Proffitt KM, Garrott RA (2007) Site fidelity of Weddell seals: the effects of sex and age. Antarct Sci 19(2):149–155
Caro TM, Hauser MD (1992) Is there teaching in nonhuman animals? Q Rev Biol 67(2):151–174
Davis RW, Wartzok D, Elsner R, Stone H (1992) A small video camera attached to a Weddell seal: a new way to observe diving behavior. In: Thomas JA, Kastelein RA, Supin AY (eds) Marine mammal sensory systems. Springer, Boston, pp 631–642
Davis RW, Fuiman LA, Williams TM, Collier SO, Hagey WP, Kanatous SB, Kohin S, Horning M (1999) Hunting behavior of a marine mammal beneath the Antarctic fast ice. Science 283(5404):993–996
Davis RW, Fuiman LA, Williams TM, Horning M, Hagey W (2003) Classification of Weddell seal dives based on 3 dimensional movements and video-recorded observations. Mar Ecol Prog Ser 264:109–122
Davis RW, Hagey W, Horning M (2004) Monitoring the behavior and multi-dimensional movements of Weddell seals using an animal-borne video and data recorder. Memoirs Natl Inst Polar Res, Spl Iss 58:148–154
Davis RW, Fuiman LA, Madden KM, Williams TM (2013) Classification and behavior of free-ranging Weddell seal dives based on three-dimensional movements and video-recorded observations. Deep Sea Res Part II 88:65–77
Dayton PK, Robilliard GA, Paine RT, Dayton LB (1974) Biological accommodation in the benthic community at McMurdo Sound. Antarc Ecol Monogr 44(1):105–128
Elliott KH, Woo KJ, Gaston AJ, Benvenuti S, Dall’Antonia L, Davoren GK (2009) Central-place foraging in an Arctic seabird provides evidence for Storer-Ashmole’s halo. Auk 126(3):613–625
Federwisch L, Owsianowski N, Richter C (2019) In-situ images of glass sponges (Porifera: Hexactinellida: Rossellidae) extracted from ROV videos recorded during POLARSTERN cruise PS82 (ANT-XXIX/9) in the Weddell Sea. PANGAEA. https://doi.org/10.1594/PANGAEA.897590
Federwisch L, Janussen D, Richter C (2020) Macroscopic characteristics facilitate identification of common Antarctic glass sponges (Porifera, Hexactinellida, Rossellidae). Polar Biol 43(2):91–110
Foster-Dyer RTN, Goetz KT, Pinkerton M, Iwata T, Holser R, Michael SA, Pritchard C, Childerhouse S, Ainley DA, Costa D, Forman J, LaRue MA (in review) Fine-scale foraging behaviours of lactating Weddell seals in Erebus Bay, Antarctica. Marine Ecol Progress Ser.
Friard O, Gamba M (2016) BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol Evol 7(11):1325–1330
Fuiman L, Davis R, Williams T (2002) Behavior of midwater fishes under the Antarctic ice: observations by a predator. Mar Biol 140(4):815–822
Fuiman LA, Madden KM, Williams TM, Davis RW (2007) Structure of foraging dives by Weddell seals at an offshore isolated hole in the Antarctic fast-ice environment. Deep Sea Res Part II 54(3–4):270–289
Goetz KT, Burns JM, Hückstӓdt LA, Shero MR, Costa DP (2017) Temporal variation in isotopic composition and diet of Weddell seals in the western Ross Sea. Deep Sea Res Part II 140:36–44
Hadley GL, Rotella JJ, Garrott RA, Nichols JD (2006) Variation in probability of first reproduction of Weddell seals. J Anim Ecol 75(5):1058–1070
Hadley GL, Rotella JJ, Garrott RA (2007) Evaluation of reproductive costs for Weddell Seals in Erebus Bay. Antarc J Anim Ecol 76(3):448–458
Heithaus M, Dill L, Marshall G, Buhleier B (2002) Habitat use and foraging behavior of tiger sharks (Galeocerdo cuvier) in a seagrass ecosystem. Mar Biol 140(2):237–248
Hounslow JL, Jewell OJ, Fossette S, Whiting S, Tucker AD, Richardson A, Edwards D, Gleiss AC (2021) Animal-borne video from a sea turtle reveals novel anti-predator behaviors. Ecology 102(4):e03251
Iwata T, Biuw M, Aoki K, Miller PJOM, Sato K (2021) Using an omnidirectional video logger to observe the underwater life of marine animals: humpback whale resting behaviour. Behav Proc 186:104369
Janussen D, Downey RV (2014) Porifera. In: De Broyer C, Koubbi P, Griffiths HJ, Raymond B, d’Udekem d’Acoz C et al (eds) Biogeographic Atlas of the southern ocean, 1st edn. Scientific Committee on Antarctic Research, Cambridge, pp 94–102
Konecki JT, Targett TE (1989) Eggs and larvae of Nototheniops larseni from the spongocoel of a hexactinellid sponge near Hugo Island. Antarc Peninsula Polar Biol 10(3):197–198
Krützen M, Mann J, Heithaus MR, Connor RC, Bejder L, Sherwin WB (2005) Cultural transmission of tool use in bottlenose dolphins. Proc Natl Acad Sci 102(25):8939–8943
Krützen M, Kreicker S, MacLeod CD, Learmonth J, Kopps AM, Walsham P, Allen SJ (2014) Cultural transmission of tool use by Indo-Pacific bottlenose dolphins (Tursiops sp.) provides access to a novel foraging niche. Proc R Soc B 281(1784):20140374
La Mesa M, Piepenburg D, Pineda-Metz SE, Riginella E, Eastman JT (2019) Spatial distribution and habitat preferences of demersal fish assemblages in the southeastern Weddell Sea (Southern Ocean). Polar Biol 42(5):1025–1040
LaRue MA, Salas L, Nur N, Ainley DG, Stammerjohn S, Barrington L, Stamatiou K, Pennycook J, Dozier M, Saints J, Nakamura H (2019) Physical and ecological factors explain the distribution of Ross Sea Weddell seals during the breeding season. Mar Ecol Prog Ser 612:193–208
Levenson DH, Ponganis PJ, Crognale MA, Deegan JF, Dizon A, Jacobs GH (2006) Visual pigments of marine carnivores: pinnipeds, polar bear, and sea otter. J Comp Physiol A 192:833–843
Lindsay AA (1937) The Weddell seal in the Bay of Whales. J Mammal 18:127–144
Lundälv T, Richter C (2019) In situ photographs of common Antarctic glass sponges (Porifera: Hexactinellida: Rossellidae) along 7 ROV profiles during POLARSTERN cruise PS77 (ANT-XXVII/3, CAMBIO) in the Weddell Sea. Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Bremerhaven, PANGAEA. 10.1594/PANGAEA.897581
Luque SP (2007) An introduction to the diveMove package. R-News 7:8–14
Lythgoe JN, Dartnall HJA (1970) A “deep sea rhodopsin” in a mammal. Nature 227:955–956
Madden KM, Fuiman LA, Williams TM, Davis RW (2008) Identification of foraging dives in free-ranging Weddell seals Leptonychotes weddellii: confirmation using video records. Mar Ecol Prog Ser 365:263–275
Marshall GJ (1998) Crittercam: an animal-borne imaging and data logging system. Marine Technol Soc: Marine Technol Soc J 32(1):11
Matsuoka K, Skoglund A, Roth G, de Pomereu J, Griffiths H, Headland R, Herried B, Katsumata K, Le Brocq A, Licht K, Morgan F (2021) Quantarctica, an integrated mapping environment for Antarctica, the Southern Ocean, and sub-Antarctic islands. Environ Model Softw 140:105015
Mellish JAE, Tuomi PA, Hindle AG, Horning M (2010) Chemical immobilization of Weddell seals (Leptonychotes weddellii) by ketamine/midazolam combination. Vet Anaesth Analg 37(2):123–131
Moll RJ, Millspaugh JJ, Beringer J, Sartwell J, He Z (2007) A new ‘view’ of ecology and conservation through animal-borne video systems. Trends Ecol Evol 22(12):660–668
Moreno CA (1980) Observations on food and reproduction in Trematomus bernacchii (Pisces: Nototheniidae) from the Palmer Archipelago. Antarctica Copeia 1980(1):171–173
Mori Y, Watanabe Y, Mitani Y, Sato K, Cameron MF, Naito Y (2005) A comparison of prey richness estimates for Weddell seals using diving profiles and image data. Mar Ecol Prog Ser 295:257–263
Nealson KH (1981) Bioluminescence: current perspectives. CEPCO Division, Burgess Publishing Company, Minneapolis, p 165
Plötz J, Bornemann H, Knust R, Schröder A, Bester M (2002) Foraging behaviour of Weddell seals, and its ecological implications. In: Arntz WE, Clarke A (eds) Ecological studies in the Antarctic sea ice zone. Springer, Berlin, Heidelberg, pp 148–156
Rotella JJ (2018) Demographic data for Weddell Seal colonies in Erebus Bay through the 2017 Antarctic field season. US Antarctic Program (USAP) Data Center. DOI: https://doi.org/10.15784/601125
Rumolo P, Zappes IA, Fabiani A, Barra M, Rakaj A, Palozzi R, Allegrucci G (2020) The diet of Weddell seals (Leptonychotes weddellii) in Terra Nova Bay using stable isotope analysis. Eur Zool J 87(1):94–104
Sato K, Mitani Y, Cameron MF, Siniff DB, Watanabe Y, Naito Y (2002) Deep foraging dives in relation to the energy depletion of Weddell seal (Leptonychotes weddellii) mothers during lactation. Polar Biol 25(9):696–702
Sato K, Mitani Y, Naito Y, Kusagaya H (2003) Synchronous shallow dives by Weddell seal mother-pup pairs during lactation. Mar Mamm Sci 19(2):384–395
Siniff DB (1981) Seal population dynamics and ecology. J R Soc NZ 11(4):317–327
Siniff DB, DeMaster DP, Hofman RJ, Eberhardt LL (1977) An analysis of the dynamics of a Weddell seal population. Ecol Monogr 47(3):319–335
Stirling I (1974) Movements of the Weddell seals in McMurdo Sound. Antarc Aust J Zool 22(1):39–43
Testa JW, Siniff DB, Ross MJ, Winter JD (1985) Weddell seal—Antarctic cod interactions in McMurdo Sound, Antarctica. Antarctic nutrient cycles and food webs. Springer, Berlin, Heidelberg, pp 561–565
Thiebot JB, Ito K, Raclot T, Poupart T, Kato A, Ropert-Coudert Y, Takahashi A (2016) On the significance of Antarctic jellyfish as food for Adélie penguins, as revealed by video loggers. Mar Biol 163(5):1–8
Watanabe Y, Mitani Y, Sato K, Cameron MF, Naito Y (2003) Dive depths of Weddell seals in relation to vertical prey distribution as estimated by image data. Marine Ecol Progress Ser 252:283–288
Watanabe Y, Bornemann H, Liebsch N, Plötz J, Sato K, Naito Y, Miyazaki N (2006) Seal-mounted cameras detect invertebrate fauna on underside of Antarctic ice shelf. Mar Ecol Prog Ser 309:297–300
Weitzner EL, Pearson LE, Tomanek L, Liwanag HE (2021) Early diving behavior in Weddell seal (Leptonychotes weddellii) pups. J Mammal 102(4):1000–1008
Wilmers CC, Nickel B, Bryce CM, Smith JA, Wheat RE, Yovovich V (2015) The golden age of bio-logging: How animal-borne sensors are advancing the frontiers of ecology. Ecology 96(7):1741–1753
Yoshino K, Takahashi A, Adachi T, Costa DP, Robinson PW, Peterson SH, Hückstädt LA, Holser RR, Naito Y (2020) Acceleration-triggered animal-borne videos show a dominance of fish in the diet of female northern elephant seals. J Exp Biol 223(5):212936
Acknowledgements
We acknowledge the mana whenua, Ngāi Tūāhuriri, on whose land this analysis and writing took place. We sincerely thank Antarctica New Zealand for looking after us in the field, the NIWA ethics team (especially Joy Coulston and Graeme Moss), Drs Akinori Takahashi and Jenn Burns for their support of the project, and Dr Mia Wege and Parker Forman for their generous guidance during data analysis and processing. The findings and conclusions in this paper are those of the author(s) and do not necessarily represent the views of the National Marine Fisheries. Service, NOAA. Mention of trade names and commercial firms does not imply endorsement by the National Marine Fisheries Service, NOAA
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. We thank our funding partners: MBIE Endeavour Fund C01X1710; NIWA SSIF (Coasts & Ocean Centre, programme 4); Ministry for Primary Industries NZ; NIWA CAPEX; Office of Naval Research (grant no. N00014-18–1-2822); NSF Antarctic Polar Programs Award 1644256; Kurita Water and Environment Foundation; Sydney Institute of Marine Science; Australian Antarctic Division; Moss Landing Marine Lab; Université Pierre et Marie Curie; Alaska Sealife Centre, Alaska; Scripps Institution of Oceanography; University of Alaska, Anchorage; University of California, Santa Cruz; Woods Hole Oceanographic Institution; National Institute of Polar Research; Massey University; and the University of Canterbury. Data collection on seal ages and breeding histories was supported by a series of grants from the National Science Foundation, Office of Polar Programs: Grant Nos. 1640481; 2147553 and prior NSF Grants to R. A. Garrott, J. J. Rotella, D. B. Siniff, and J. Ward Testa.
Author information
Authors and Affiliations
Contributions
Author contributions were as follows: KG & MP contributed to the study conception/design; KG, MP & DC contributed to funding acquisition; RFD, KG, TI, RH, CP, SC, SM & JR contributed to fieldwork and/or data gathering; RFD, KG, TI, MP, LF & ML contributed to data analysis and interpretation; figures and the first draft of the manuscript were prepared by RFD; all authors commented on previous versions of the manuscript; all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Ethical approval
All procedures involving animals were approved by the New Zealand Department of Conservation (permit number DOC-69331-MAR), Ministry of Foreign Affairs and Trade, and NIWA’s Animal Ethics Panel (Project number AEC210 (END18301/Ross-RAMP: Ross Sea Research and Monitoring Program)).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (MP4 13602 KB)
Supplementary file3 (MP4 18121 KB)
Supplementary file4 (MP4 7215 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Foster-Dyer, R.T.N., Goetz, K.T., Pinkerton, M.H. et al. First observations of Weddell seals foraging in sponges in Erebus Bay, Antarctica. Polar Biol 46, 611–621 (2023). https://doi.org/10.1007/s00300-023-03149-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-023-03149-1