Abstract
Endemic Antarctic macroalgae are especially adapted to live in extreme Antarctic conditions. Their potential biogeographic distribution niche is primarily controlled by the photoperiodic regime and seawater temperatures, since these parameters regulate growth, reproduction, and survival during the entire life cycle. Here we analyzed the upper survival temperature (UST) of juvenile sporophytes and the temperature range for sporophyte formation from gametophytes of Desmarestia menziesii, one of the dominant endemic Antarctic brown algal species. This process is a missing link to better evaluate the full biogeographical niche of this species. Two laboratory experiments were conducted. First, growth and maximum quantum yield of juvenile sporophytes were analyzed under a temperature gradient (0, 5, 10, 12, 13, 14, 15, and 16 °C) in a 16:8 h light:dark (LD) regime (Antarctic spring condition) for 2 weeks. Second, the formation of sporophytes from gametophytes (as a proxy of gametophyte reproduction) was evaluated during a 7 weeks period under a temperature gradient (0, 4, 8, 12, and 16 °C), and two different photoperiods: 6:18 h LD regime simulating winter conditions and a light regime simulating the Antarctic shift from winter to spring by gradually increasing the light period from 7.5:16.5 h LD (late winter) to 18.5:5.5 h LD (late spring). Sporophytes of D. menziesii were able to grow and survive up to 14 °C for 2 weeks without visible signs of morphological damage. Thus, this species shows the highest UST of all endemic Antarctic Desmarestiales species. In turn, gametophyte reproduction solely took place at 0 °C but not at 4–8 °C. The number of emerging sporophytes was six times higher under the light regime simulating the transition from winter to spring than under constant short day winter conditions. There was a negative relationship between the number of sporophytes formed and the gametophyte density at the beginning of the experiment, which provides evidence that gametophyte density exerts some control upon reproduction in D. menziesii. Results strongly indicate that although sporophytes and gametophytes may survive in warmer temperatures, the northernmost distribution limit of D. menziesii in South Georgia Islands is set by the low temperature requirements for gametophyte reproduction. Hence, global warming could have an impact on the distribution of this and other Antarctic species, by influencing their growth and reproduction.
Similar content being viewed by others
Introduction
Since more than 40 million years, Antarctica has been isolated through the Antarctic convergence after the opening of the Drake Passage (Scher and Martin 2006). This separation from southern America has configured its environmental conditions, with sea-surface temperatures constantly below 3.5 °C in summer (Knox 1968, 1970). The seawater temperature in the Antarctic region is one of the most stable on the planet (Wiencke et al. 2007), but other factors such as the strong seasonality of light are highly variable (Wiencke and Clayton 2002). Photoperiod, irradiance, and temperature are the main abiotic factors that regulate growth, reproduction, and survival of Antarctic macroalgae (Zacher et al. 2009). As a result of the long exposure to these special environmental conditions, which were rather constant over geological time scales, endemism in Antarctica is very high compared to other regions of the world with 35.3% of the brown Antarctic macroalgae being endemic (Oliveira et al. 2020).
The brown algal order Desmarestiales is the biomass dominant group around Antarctica and forms three-dimensional underwater forests similar to kelp forests in temperate to Arctic zones around the world (Wiencke and Amsler 2012; Quartino et al. 2020). It includes five endemic species, Desmarestia menziesii J. Agardh, D. antarctica R.L. Moe and P.C. Silva, D. anceps Montagne, Himantothallus grandifolius (Gepp and Gepp) Zinova, and Phaeurus antarcticus Skottsberg. All of them are ecologically important for their biomass, coverage and as bioengineering species (Clayton 1994; Amsler et al. 1995; Quartino et al. 2005, 2013; Quartino and Boraso de Zaixso 2008). Desmarestiales exhibit a heteromorphic life cycle with alternating generations between a macroscopic sporophyte and a microscopic gametophyte (Ramirez and Peters 1992) and each stage has specific requirements for growth and development as known from macroalgae in general (Floc’h et al. 1991; Gómez and Wiencke 1996). The life cycle of Antarctic Desmarestiales is strictly controlled by the seasonal course of daylength and temperature. Under laboratory conditions gametophytes become fertile under short day conditions, such as has been shown for H. grandifolius, D. anceps and D. menziesii and sporophytes begin to grow at the end of winter and have their highest growth rate in spring (Wiencke 1990). Maximal survival temperatures of D. anceps, D. antarctica, H. grandifolius, and P. antarcticus sporophytes are between 11 and 13 °C which is 8 to 10 °C less than in Arctic sister species (Wiencke et al. 1994). The UST of Antarctic Desmarestiales microscopic gametophytes are slightly higher than for the respective sporophytes and range between 13 and 16 °C (Wiencke and tom Dieck 1989). The optimal growth temperatures of these species are considerably lower than their survival temperatures and vary between 0 and 5 °C (Wiencke et al. 1994). This low temperature growth optimum of sporophytes is a special adaptation to Antarctic conditions, not present elsewhere in macroalgae of other biogeographical zones (Wiencke et al. 1994). The growth optimum of gametophytes is higher and wider than of sporophytes and ranges between 0 and 15 °C (Wiencke and tom Dieck 1989; Wiencke et al. 1994).
Temperature performance of all Antarctic endemic Desmarestiales species but D. menziesii is well known (Wiencke and tom Dieck 1990). Its temperature windows for sporophyte growth and survival and for gametophyte reproduction are crucial to explain the species’ biogeographical distribution limits, but are still unknown. D. menziesii is particularly abundant at the Antarctic Peninsula and adjacent islands but stretches between the Ross Sea (76°S) in the south (Wiencke et al. 2014; Küpper et al. 2019) and South Georgia Islands (54°26′S, 36°33′W) which is the northernmost locality of occurrence (Davenport et al. 1996; Wiencke and Clayton 2002). South Georgia Islands serve as a bridge between two phytogeographic regions inheriting Antarctic and sub-Antarctic biota, and represents the northern biogeographical distribution limit of many benthic endemic Antarctic species (Hogg et al. 2011). On these islands the photoperiod varies between 7.4 h light in June and 17 h in December (Haderspeck and Hoffmann 1990) and the mean sea-surface temperature (SST) between 1972 and 2006 annually changed between 0–1.5 °C in August to November and 3–4 °C in February (Barnes et al. 2006). The next or nearest possible landmass suitable for macroalgae, further north and west, is either Falkland Islands / Islas Malvinas (51°48′S, 59°31′W) or the southern tip of South America (55°02′S, 66°32′W), where SST are always above 4 °C (Balestrini et al. 1998; Arkhipkin et al. 2004). As the species is absent here our hypothesis is that the life cycle of D. menziesii is characterized by one process that is restricted to temperatures below 4 °C. There is one doubtful record of D. mensiezii for Falkland Islands/Islas Malvinas (Papenfuss 1964) that has not been verified (Ramirez 2010) and there are no herbarium records of D. menziesii for these Islands in the National History Museum of London (MN Clayton, J Brodie, pers. comm.).
Wiencke and tom Dieck (1990) observed that female gametophytes of D. menziesii grow between 0 and 10 °C with an optimum at 5 °C and their UST was 16–17 °C. In general, sporophytes of the Antarctic Desmarestiales species have a lower UST than their gametophytes (Wiencke and tom Dieck 1989, 1990). We therefore predicted the following: (I) the UST of D. menziesii sporophytes also is 2–3 °C lower than that of gametophytes. As density of brown algae gametophytes (Reed 1990; Reed et al. 1991; Wiencke et al. 1995; Choi et al. 2005; Carney and Edwards 2010) and photoperiod are two major factors determining gametogenesis in kelps and Desmarestiales (Hsiao and Druehl 1971; Wiencke 1990; Wiencke et al. 1995; Choi et al. 2005), we expected (II) higher sporophyte formation at lower gametophyte densities and an effect of photoperiod on sporophyte formation. Under laboratory conditions, gametophytes became fertile and formed juvenile sporophytes in early winter to spring conditions (Wiencke et al. 1995). Thus, we expected (III) a higher formation of sporophytes under fluctuating photoperiods simulating winter to spring compared to continuous austral winter short daylengths. Lastly, as the optimal growth temperature of D. menziesii gametophytes is 5 °C (Wiencke and tom Dieck 1990) and the highest SST at its northern distribution limit is < 4 °C (Hogg et al. 2011), we predicted (IV) that the optimal temperature for formation of sporophytes is below 5 °C.
In order to verify these expectations, we experimentally determined the growth capacity and survival of sporophytes of D. menziesii along a temperature gradient between 0 and 16 °C and quantified sporophyte formation from gametophytes along a gametophyte density gradient and along a temperature gradient between 0 and 16 °C in combination with relevant ecological photoperiodic conditions.
Materials and methods
In order to assess the UST of the Antarctic endemic brown alga D. menziesii, laboratory experiments were set-up, exposing juvenile cultured sporophytes to increasing temperatures (0 °C to 16 °C, experiment 1a + b). Another set of experiments was performed on gametophytes of D. menziesii in order to assess growth and sporophyte formation related to temperature, photoperiod, and gametophyte density (experiment 2a + b). Algal material of D. menziesii was grown from unialgal male and female clonal gametophyte stock cultures available at the Alfred Wegener Institute (AWI, culture number: 3103 and 3012—male and female). The gametophytes were originally isolated from spores of fertile sporophytes collected at Ardley Bay (King George Island/Isla 25 de Mayo; South Shetland Islands, Antarctica) in 1986. Cultures were grown in Provasoli-enriched natural sterile seawater (PES; Provasoli 1968). As stock cultures have been kept for > 30 years at 0 to 5 °C, we cannot exclude that slight adaptations to culture conditions may have occurred or that the temperature priming may have had an effect on the results (see Liesner et al. 2020). Recent work on kelp gametophytes has however shown that long-term storage of clonal gametophytes did not alter the overall response to temperature even after 25 years (Martins et al. 2019).
Experiment 1: Upper survival temperature of Desmarestia menziesii sporophytes
Vegetative male and female gametophytes of D. menziesii were carefully ground with pestle and mortar, and grown together at 0 °C in order to induce fertilization and the formation of sporophytes after the method of tom Dieck (1992). Over ~ 2 years the sporophytes were grown at 0 °C and under low irradiance conditions (~ 10 µmol photons m−2 s−1), in a 12:12 h light:dark (LD) regime (OSRAM L36W/965, Biolux, München, Germany). The culture medium (PES) was exchanged in regular intervals to avoid nutrient depletion. Four months prior to the experiment young sporophytes were separated into 5 L aerated glass bottles and grown at 0 °C under 30 µmol photons m−2 s−1 and a 16:8 LD regime. The used irradiance and photoperiod enables high growth rates in Antarctic Desmarestiales according to Wiencke (1990).
For the experiment, 52 individuals of similar size (~ 6 cm) were selected. Two sporophytes each were subjected into 4 replicate 2-L glass beakers per temperature (n = 4; day -6) and subjected to an acclimation period (Fig. 1). The acclimation took place in 5 °C steps every two days, beginning at 0 °C (Fig. 1). After the acclimation, one of the two sporophytes per replicate was removed for analysis of maximum quantum yield (Fv/Fm), fresh weight (FW), and dry weight (DW); the other sporophyte was exposed to the target temperature for 2 weeks and was subjected to the same parameter measurements (Fig. 1). Two consecutive experiments were performed (experiments 1a and 1b). In experiment 1a, sporophytes were grown in a temperature gradient between 0 and 14 °C (Fig. 1). As sporophytes survived 14 °C without damage for 2 weeks, a consecutive experiment (experiment 1b) was performed under otherwise identical conditions (Fig. 1). At day -6, four specimens from the stock of sporophytes were randomly selected to analyze the initial condition (Fv/Fm, FW and DW) of the sporophytes in both experiments (exp 1a and 1b).
The temperature gradient was established in a walk-in culture room (0 °C) in water baths tempered by thermostats (Thermo Haake DC 10 and Huber Variostat CC + Pilot ONE, Peter Huber Kältemaschinen GmbH, Offenburg, Germany). Temperatures were set to 0, 5, 10, 12, 13, 14 °C (experiment 1a) and to 15 and 16 ºC (experiment 1b). Irradiance was kept at 30 µmol photons m−2 s−1 (OSRAM L36W/965, Bioloux, München, Germany) measured at the water surface using a LI-COR LI-250A Light Meter (LI-COR, Inc., Lincoln, USA) in a 16:8 LD regime. The two sporophytes per replicate had an initial fresh weight of 0.227 ± 0.064 g (n = 4) and 0.242 ± 0.076 g (n = 4) (exp. 1a and 1b, respectively; p = 0.557, t-test). During the experiments, the culture medium (PES) was exchanged once a week and each beaker was aerated with artificial air containing 380 ppm of CO2.
Fresh weight measurement of the sporophytes
Fresh weight of sporophytes was estimated before the acclimation (day -6), at the start of the experimental phase (day 0), on days 4, 7, 11, and 14 (end of the experiment; Fig. 1). Each sporophyte was carefully blotted dry with tissue paper before weighing (Sartorius CPA 323S, Göttingen, Germany). After weighing, the sporophytes were placed back into the beaker. The relative growth rate (RGR) on the base of FW was calculated according to the following formula:
where N0 is the initial fresh weight, Nt is the fresh weight at day t, and T = time interval in days.
Maximum quantum yield (F v/F m) of the sporophytes
Maximum quantum yield was measured as variable fluorescence of PSII using a PAM 2100 (Walz GmbH, Effeltrich, Germany). Although maximum quantum yield primarily has been used to observe irradiance stress, temperature as a physiological stressor also affects photosynthesis in macroalgae (Navarro et al. 2020) and Fv/Fm has been successfully used to quantify detrimental responses to temperature as an early warning indicator rather than thallus bleaching, especially at upper sub-lethal temperatures (Olabarria et al. 2013; Graiff et al. 2015; Savaglia et al. 2019). Prior to the beginning of the acclimation phase (day -6), pre-experimental individuals were measured to document the initial status of the material after cultivation (n = 4). Later on measurements took place on experimental day 0, 1, 4, 7, 11, and 14 (Fig. 1). The PAM fiber optic was placed at 1–2 cm from the apical tip of the thallus between the main axis and the first branch and Fv/Fm was determined after 3 min of dark adaption following Zacher et al. (2016): darkening for 3, 5, 7, 10, and 15 min did not result in any significant differences in Fv/Fm and values as high as in other studies were reached (Rautenberger et al. 2015; Schoenrock et al. 2015; Savaglia et al. 2019). Maximum quantum yield of PSII (Fv/Fm) was calculated by the PAM software, according to the following equation:
where F0 is the minimal fluorescence of dark-adapted sample, Fm is the maximum fluorescence of dark-adapted sample.
Experiment 2: Growth and reproduction of Desmarestia menziesii gametophytes under different temperatures, photoperiods, and gametophyte densities
Experiment 2 was performed in order to study the effect of temperature in increasing spring daylengths (experiment 2a) and the effect of different gametophyte densities (experiment 2b) on growth of D. menziesii gametophytes and their ability to form sporophytes. Equal parts of vegetative male and female gametophytes of D. menziesii were mixed and carefully ground with pestle and mortar, and then filled up with PES. After 24 h in darkness at 0 °C, gametophytes were used for two different experimental set-ups. According to Wiencke (1990), Desmarestiales gametophytes grow at low photon fluence rates and light saturation occurs between 4 and 12 μmol photons m−2 s−1. Moreover, Gomez and Wiencke (1996) indicated that gametophytes and young sporophytes of D. menziesii perform photosynthesis better under low light conditions and required much lower photon fluence rates than adult sporophytes for growth. In order not to generate additional stress and to provide the best conditions, the experiment was carried out at low photon fluence rates of 10 µmol photons m−2 s−1.
Experiment 2a: Sporophyte formation in a temperature gradient and changing photoperiod
To assess the formation of sporophytes in a temperature gradient, 350 µl of the gametophytic stock solution was sown in plastic Petri dishes (89 × 35 mm) and diluted with 100 ml of PES. The initial density of 10 randomly Petri dishes were counted, resulting in a density of 870 ± 209 (n = 10) gametophytes cm−2. Five petri dishes each (n = 5) were subjected to a temperature gradient (0, 4, 8, 12, and 16 °C). Temperatures were controlled in water baths equipped with thermostats (Thermo Haake DC10, Karlsruhe, Germany, for 4 to 16 °C); 0 °C was the temperature of a walk-in culture room. Culture medium (PES) was exchanged bi-weekly. Before starting the experiment, the gametophytes were acclimated for 6 days to increasing temperatures starting from 0 °C. Temperature was increased by 4 °C every 2 days until reaching the target temperature. Gametophytes were exposed for 7 weeks to the experimental temperatures. Daylength increased week by week starting in short day conditions (7.5:16.5 h LD) and ended in long day conditions (18.5:5.5 h LD) (SD → LD), thereby simulating the change in daylength from winter to spring in Antarctica (Wiencke 1990).
Experiment 2b: Sporophyte formation under different gametophyte densities
To assess the formation of sporophytes under a gametophyte density gradient, three different aliquots of the gametophytic stock solution (200, 350, and 500 µL) were sown into plastic Petri dishes and diluted with 100 mL of PES (n = 5). The density of gametophytes in the 350 µL aliquot served as the 100% treatment (d100; 870 ± 209 (n = 10) gametophytes cm−2, the same as used in experiment 2a). After sowing, the other two densities were calculated in relation to the d100 treatment (Table 1). Gametophyte development was followed at 0 °C, over 7 weeks in constant short days of 6:18 LD simulating Antarctic winter daylenghts. The amount of light in hours was approximately half to that applied in experiment 2a (Table 2).
Approximately every 10 days the gametophyte development in both experiments was monitored under an inverted microscope (Olympus CKX41, with a Canon EOS 550D camera adapted by LM Scope, Graz, Australia) to check qualitatively if sporophytes developed. After 7 weeks, the emerging young sporophytes were counted. Afterward, the DW of all algal material in each Petri dish (gametophytes plus developing sporophytes) of both experiments was determined as proxy for overall growth. The complete algal material was filtered on pre-dried and weighed GF/F filters (0.7 µm; Whatman), and dried at 60 °C for 48 h and weighed again.
Statistical analysis
Experiment 1
We tested for differences in initial (day-6) FW and Fv/Fm with a one-way ANOVA for experiment 1a and 1b together. FW and Fv/Fm were similar among the different treatments (one-way ANOVA: F7,24 = 0.55, p = 0.78 and F7,24 = 0.9, p = 0.52, respectively). Because none of the tested parameters showed significant differences prior to the start of the acclimation phase, we combined the data of both experiments for further statistical analysis.
Our experimental data were obtained from individuals that were measured repeatedly through time in case of growth and Fv/Fm. Linear and non-linear mixed-effect models are particularly useful when there is temporal pseudo-replication (repeated measurement) (Pinheiro and Bates 2000). Therefore, we used linear mixed-effect models (LME) in order to include each sporophyte as a random effect and thus accounting for within-individual correlation in all models (Littell et al. 2005). Via LME we determined if growth and maximum quantum yield of D. menziesii sporophytes (RGR and Fv/Fm, dependent variables) varied over time and the temperature gradient (independent variables). A model validation was applied to verify that underlying statistical assumptions were not violated. Normality of residuals was assessed by plotting theoretical quantiles versus standardized residuals (Q–Q plots). Homogeneity of variance was evaluated by plotting residuals versus fitted values, and influential data points were identified using Cook's distance method (Quinn and Keough 2002). The validation procedure showed that there was no evidence of nonlinearity, but for all models, the diagnostic residual plots indicated heteroscedasticity, due to the inherent heterogeneity of variance within independent variables. In order to obtain homogeneity of variance, we used LME models with a variance-covariate structure (Zuur et al. 2007). Thus, a set of models with different variance structures were compared with the equivalent model without the LME (“nlme” package, Pinheiro et al. 2015) extension, using Akaike's Information Criterion (AIC) and examination of plots of residuals versus fitted values. Finally, where necessary, we conducted mean comparisons using an interaction means test in the “emmeans” package (Russell 2020) of the R-environment.
Experiment 2
The DW of gametophytes (experiment 2a and b) and the number of sporophytes cm−2 (experiment 2b only) were analyzed with one-way ANOVAs for each experiment separately. When the ANOVA revealed significant differences, a post hoc Tukey’s honest significant difference test was applied. T-tests were applied to compare DW and the number of sporophytes cm−2 of the same density (d100) and temperature (0 °C) with those of the variable irradiance conditions (experiment 2a vs. 2b). Before performing the statistical analysis, normality was checked with the Shapiro–Wilk test and homogeneity of variances was verified using the Levene’s test.
All statistical analyses were conducted in R 3.4.4 (R Development Core Team 2018). Graphics were generated with the ggplot2 package (v. 3.1.1).
Results
Experiment 1: Growth and survival along a temperature gradient in Desmarestia menziesii sporophytes
Growth and upper survival of D. menziesii sporophytes was determined in a 14 days experiment between 0 and 16 °C. The species grew and survived between 0 and 14 °C without any apparent visual damage, while sporophytes at 15 °C showed first signs of degradation and at 16 °C bleached necrotic apices.
Relative growth rate (RGR)
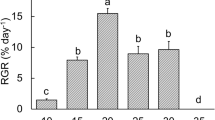
Temperature significantly affected the growth of the sporophytes (Table 3). The highest RGR of D. menziesii were at 0 and 5 °C (1.24 ± 0.23 and 1.11 ± 0.29 g g−1 FW day−1, n = 4, respectively; ESM1), and RGR significantly decreased at higher temperatures (Fig. 2, Table 3). At 16 °C, after 7 days the RGR showed negative values (− 0.19 ± 0.38 g g−1 FW day−1, n = 4, ESM1) and the overall growth rate (14 days) was negative (− 0.003 ± 0.15 g g−1 FW day−1, n = 4, ESM2). Although RGR at 12 and 13 °C was not significantly different from 5 °C, there was a trend that RGR at 0 and 5 °C was higher than at warmer temperatures (10 to 16 °C).
Predicted values of mean (from the linear mixed model) of the relative growth rate (RGR) of Desmarestia menziesii sporophytes along a temperature gradient over 14 days (mean ± SE, n = 4; output from the linear mixed modeling, see statistics). Lower case letters indicate significant differences of the post hoc test between different temperature treatments
Maximum quantum yield
Maximum quantum yield (Fv/Fm) of D. menziesii sporophytes measured over the course of the 14 days experiment was significantly affected by the interaction between temperature and time (Table 3). Throughout time Fv/Fm remained constantly high at 0 and 5 °C (0.63 ± 0.01 and 0.68 ± 0.01, n = 4, respectively) (Fig. 3 and ESM3), while Fv/Fm significantly decreased at higher temperatures. At 15 and 16 °C, Fv/Fm was significantly lower than in all other temperatures (0.42 ± 0.06 and 0.27 ± 0.06, n = 4 on day 14, respectively, Fig. 3, Table 3). The post hoc test showed a higher and significantly decreasing slope at warm temperatures (13, 15 and 16 °C) with the exception of 14 °C that was similar to 5 to 12 °C (Fig. 3, Table 3). Generally, the slope of the maximum quantum yield over time showed a gentle decrease between 10 and 14 °C and dropped sharply at higher temperatures of 15 and 16 °C (Fig. 3), while it was constant at 0 and 5 °C.
Maximum quantum yield (Fv/Fm) of Desmarestia menziesii sporophytes along a temperature gradient over 14 days of exposure. Lines are predicted values of mixed linear model of the maximum quantum yield at different temperatures (differentiated with different colors) in relation to time of exposure and normalized by day 0 values. Predicted slope values of the mixed linear model are visualized by the points in the inset panel. Lower case letters indicate significant differences of the post hoc test between different temperature treatments
Experiment 2: Growth and reproduction of Desmarestia menziesii gametophytes under different temperatures, photoperiods, and gametophytes densities
Experiment 2a
In this experiment the final biomass of gametophytes and emerging sporophytes in a temperature gradient between 0 and 16 °C was investigated as a proxy for growth over 7 weeks. Temperature significantly affected the final biomass, being higher at 4 and 8 °C than at lower (0 °C) and higher (12 and 16 °C) temperatures (Fig. 4, Table 4, Tukey post hoc test, p < 0.0001). Gametophytes at 4 and 8 °C grew strongly, which was also macroscopically visible (Fig. 5). Some dead cells were visible under the microscope at 12 °C, while only few gametophyte cells were still alive at 16 °C. Interestingly, sporophytes of D. menziesii only developed at 0 °C and none at higher temperatures. Therefore Fig. 6 only shows the number of sporophytes of experiment 2a at 0 °C against the results of experiment 2b (gametophyte density gradient).
Biomass of Desmarestia menziesii gametophytes and emerging young sporophytes after 7 weeks in a combined temperature and irradiance gradient (experiment 2a; for details of daylengths see “Materials and methods” section) and in a gametophyte density gradient at 0 °C under inductive short daylengths (experiment 2b). Values are means ± SD (n = 5). Lower case letters indicate significant differences in DW among temperatures (exp 2a) and capital letters indicate significant differences of the two d100 density treatments which were either cultivated in changing daylengths (exp 2a) or short day conditions (exp 2b). See Table 4 for results of statistical analysis
Number of young sporophytes of Desmarestia menziesii after 7 weeks in different photoperiods and gametophyte densities at 0 °C. Experiment 2a (left) and 2b (right). Boxes represent the upper and the lower quartile, the line is the median, whiskers indicate maximum and minimum values (excluding outliers). Lower case letters indicate significant differences in number of sporophytes cm−2 among the different densities; and capital letters indicate significant differences between the same densities subjected to different photoperiod conditions (d100, Exp 2a vs 2b)
Experiment 2b
In experiment 2b, final DW as a proxy for growth was determined after 7 weeks starting from three different initial gametophyte densities, all exposed to 0 °C and constant short daylengths (simulated winter condition). There were no significant differences in final DW between the different density treatments (Fig. 4, Table 4). Although sporophytes were formed under all three densities, the final sporophyte density was significantly higher at the lowest density treatment (d60) compared to the highest density treatment (d140) (Fig. 6, Table 4; Tukey post hoc test, p = 0.024).
Comparison of growth in the gametophyte density d100 at 0 °C subjected to either fluctuating daylength (experiment 2a) or short day (SD, experiment 2b) showed a significant effect of daylength conditions on DW and the formation of sporophytes (t-test, n = 5, p < 0.0009, 4.17 ± 0.4, 2.32 ± 0.6 mg and n = 5, p < 0.001, 120 ± 16.3, 21.58 ± 6.8, respectively, Figs. 4 and 6). Dry weight was approximately twice as high under increasing daylength (experiment 2a) compared to the constant SD condition (experiment 2b) (Fig. 4), while the number of sporophytes was approx. six times higher at 0 °C in the increasing daylength (experiment 2a) compared to the same gametophyte density in the constant SD condition of experiment 2b (Fig. 6).
Discussion
The current study on the Antarctic endemic brown macroalgae D. menziesii supports the general stenothermic character known of Antarctic Desmarestiales species (Wiencke et al. 2014). It is striking however that sporophytes of D. menziesii grew optimally between 0 and 5 °C, but survived at 14 °C, over 2 weeks. Although the upper temperature limit of survival is higher than reported for other Antarctic Desmarestiales species (Wiencke et al. 2014), the pronounced cold-stenothermal character is further characterized by the narrow temperature window for formation of sporophytes. Sporophytes were only formed at 0 °C but not at ≥ 4 °C. The observed inability of gametophytes to form sporophytes at temperatures ≥ 4 °C might also be a matter of gametophyte density rather than alone a temperature effect as gametophyte density considerably influenced sporophyte formation at 0 °C. As intermediate low temperatures have not been tested, it cannot be excluded that fertility may take place between 0 and < 4 °C. Our study was intended to generate missing data for explaining the control of the life cycle by temperature and not its physiological base. However, it is known that temperature directly and indirectly influences macroalgae biology, from subcellular to community-level processes (Navarro et al. 2016). Specifically, as higher temperatures accelerated growth of gametophytes and thereby density of gametophyte cells increased considerably, the observed reduction or inhibition of gametophyte reproduction may have been regulated, e.g., by hormonal secretion, as suggested by Ebbing et al. (2020). There are also ecological explanations: suppressed reproduction when density of the gametophytes is high may benefit offspring, avoiding a high density of sporophytes competing for light and space (Dayton et al. 1984).
In addition, we showed that daylength is crucial for sporophyte formation. Application of increasing daylengths characterizing spring conditions in Antarctica had a superior positive effect on sporophyte formation compared to constant short daylengths simulating Antarctic winter photoperiods. This further supports the hypothesis that D. menziesii is a season anticipator (Kain 1989) and in nature probably mostly recruits in spring. Since gametophytes were growing considerably better at 4 and 8 °C compared to 0 °C, future warming temperatures may enhance the production of the gametophyte seed-bank that is assumed to exist in nature (e.g., Robuchon et al. 2014; Küpper et al. 2016). This might be positive for the spread and development of D. menziesii, but only if there are sufficiently long periods of cold temperatures in short daylengths that are needed for gametangium formation and thereby initiation of sporophytes (Wiencke et al. 1995).
Response of Desmarestia menziesii sporophytes along a temperature gradient
In this study, the UST of D. menziesii sporophytes was investigated for the first time. The species grew and survived up to 14 °C before thallus deterioration started and confirms our hypothesis (I) that the UST of sporophytes is a few degrees lower than that of gametophytes which survive 16–17 °C (Wiencke and tom Dieck 1989, 1990). Thus, D. menziesii exhibits the highest UST of the five Antarctic endemic Desmarestiales species which otherwise only survive 11–13 °C (Wiencke et al. 2014). Only those Antarctic species with a wider distribution zone spanning from Antarctica to cold-temperate southern America, such as Adenocystis utricularis, Desmarestia confervoides, and Acrosiphonia arcta, show much higher UST, between 18 and 26.4 °C (Peters and Breeman 1993; Wiencke et al. 2014).
As growth was optimal at 0–5 °C, D. menziesii otherwise is in line with other endemic Antarctic Desmarestiales (Wiencke and tom Dieck 1989, 1990; Zacher et al. 2016; Savaglia et al. 2019) showing a specific cold-water adapted growth response. At higher temperatures growth and maximum photosynthetic quantum yield Fv/Fm of sporophytes constantly decreased over a period of 14 days, reaching lowest values at 15 and 16 °C. The drastic decrease in Fv/Fm > 14 °C corresponds well with the general stress response of the sporophytes which visually degraded at 15–16 °C after 14 days, and overall growth rates became negative. But in contrast to growth photosynthetic efficiency Fv/Fm had a wider temperature range showing quite stable high values up to 14 °C over 2 weeks while growth decreased by more than 50% from 10 °C onwards. A positive physiological effect of warmer temperatures on the photosynthetic response had already been demonstrated in short-term 2 day-experiments at 7 °C for D. menziesii and other Antarctic Desmarestiales that was especially prominent when the species were additionally stressed by UV irradiation (Rautenberger et al. 2015).
Response of gametophytes under a set of temperatures, daylenghts, and densities
Our results show that reproduction of gametophytes of D. menziesii seems to be restricted to 0 < 4 °C and is enhanced under photoperiods changing from short to long daylength and thereby simulating Antarctic spring conditions. Despite the large number of studies on temperature tolerance of Antarctic algae species, summarized in Wiencke et al. (2014), the temperature requirement for reproduction is scarce, especially for endemic Desmarestiales species. In case of D. antarctica gametogenesis took place between 0 and 5 °C (as Desmarestia sp. in Wiencke and tom Dieck 1989), but the temperature required for sporophyte formation is still unknown. In the present study D. menziesii was able to reproduce and form sporophytes only at 0 °C and not at 4 °C or higher temperatures, although the optimum growth range of female D. menziesii gametophytes is between 0 and 10 °C (Wiencke and tom Dieck 1990). We thereby supported hypothesis (IV) that the temperature range for gametophyte fertility of D. menziesii is lower than for optimum growth of gametophytes. It is important to highlight that the warmer temperatures of 4 and 8 °C notably improved the vegetative gametophyte growth, whereas reproduction was unsuccessful and there was no sporophyte formation.
Other Antarctic Desmarestiales gametophytes had their optimal growth in winter but the season for reproduction slightly varied among species. While D. anceps and H. grandifolius only reproduced successfully in short days simulating the Antarctic winter photoperiod, Phaeurus antarcticus became fertile under winter to spring fluctuations of the photoperiod (Wiencke 1990). Thus, D. menziesii responds similarly to P. antarcticus, as our study shows that sporophyte formation increases in spring conditions compared to Antarctic winter conditions supporting hypothesis (III). In winter daylight conditions (constant 6:18 h LD) the formation of sporophytes was only 16% of the production in spring conditions. This difference may be the result of photoperiods per se which act as developmental triggers (Brawley and Johnson 1992) or might be a result of the overall difference in total light or a combination of both. For example, in Arctic endemic Laminaria solidungula, gametogenesis was induced in darkness and very short daylengths between 1 and 7 h light but not in long days or night break conditions (5:9:1:9 LDLD). The subsequent formation of sporophytes was considerably enhanced if short days were followed by long days (tom Dieck 1989). As this was best in the combination of very low short day lengths (1:23 LD) with long days, a mere photosynthetic effect could be excluded. On the other hand, both photoperiodic conditions in our experiments differed in light hours over 7 weeks by 200%. The difference in sporophyte formation output in D. menziesii was however 600%, also indicating that sporophyte formation probably was not only dependent on increased photosynthesis. Although we did not test light quality during our experiments, at least kelp gametophytes need blue light for induction of gametogenesis (Lüning and Dring 1975; Brawley and Johnson 1992; Lüning 1994). As Desmarestiales and Laminariales are sister orders (Tan and Druehl 1996), blue light should always be considered also for Desmarestiales as a possible source of control. As the amount of blue quanta in the increasing daylength condition was much higher than in the constant shortday treatment it may have affected gametogenesis of D. menziesii and contributed to the observed differences in the number of formed sporophytes.
But it is more probably that the considerable growth of gametophytes at 4 and 8 °C had a negative influence on gametogenesis. The high gametophyte biomass was a result of vegetative growth of gametophytes increasing the overall gametophyte cell density. Even if no gametophyte fragmentation took place (which was not monitored), the number of potential reproductive cells thereby increased (Ebbing et al. 2020). In order to better understand whether the inhibition of gametogenesis at 4 and 8 °C was due to temperature or density, we investigated the effect of gametophyte density on sporophyte formation just at inductive 0 °C and under winter daylength conditions (6:18 h LD). Formation of sporophytes was clearly dependent on the initial gametophyte density: the lower the initial gametophyte density the higher the formation of sporophytes. This is in accordance with hypothesis (II) that the formation of sporophytes is positively related to a low gametophyte density. The results are in line with studies on kelp species, such as Pterygophora californica Ruprecht, Macrocystis pyrifera (Linnaeus) C.Agardh, Undaria pinnatifida (Harvey) Suringar and Saccharina latissima (Linnaeus) C.E.Lane, C.Mayes, Druehl and G.W.Saunders, where reproduction was negatively correlated to gametophyte density (Reed 1990; Reed et al. 1991; Choi et al. 2005; Ebbing et al. 2020).
Previous studies on the life cycles of the order Desmarestiales have shown that the reproduction and development of gametophytes and sporophytes are seasonally regulated (Anderson and Bolton 1989; Ramirez et al. 1986). Even, Wiencke (1990) observed that phenology is mainly regulated by the seasonal light regime for the Antarctic Desmarestiales algae. Moreover, our results showed that seawater temperature and gametophyte density are also important factors for regulating the reproduction of the endemic Antarctic alga D. menziesii.
Geographic distribution of Desmarestia menziesii
Desmarestia menziesii has the widest geographical distribution of Antarctic Desmarestiales, with southernmost records in the Ross Sea at 76°S (Wiencke et al. 2014; Küpper et al. 2019; Oliveira et al. 2020) and the northernmost occurrence reported from South Georgia Islands (54°S) (John et al. 1994; Wiencke and Clayton 2002; Wells et al. 2011). Therefore, in the early 1990s, D. menziesii was classified as a species with an Antarctic to cold temperate imprint (Wiencke and tom Dieck 1989, 1990; Wiencke et al. 1994) along with two red algae, I. cordata and Sarcopeltis antarctica (as Gigartina skottsbergii Setchell and N.L. Gardner). The two red algae had optimal growth rates at 0–5 °C, typical for endemic Antarctic species, but also rather high USTs of 15–16 °C (Wiencke and tom Dieck 1990; Wiencke et al. 1994). This led to the conclusion that this UST probably is indicative also for a cold-temperate character, especially as also South American populations were known. Meanwhile I. cordata and S. antarctica from Antarctica are differentiated by molecular methods from South American populations and are considered true and separate Antarctic endemic species (Hommersand et al. 2009; Billard et al. 2015; Ocaranza-Barrera et al. 2019; Hughey et al. 2020). The combination of a low optimal growth temperature and a relatively low UST are thus intrinsic characters of Antarctic endemic species, as was also verified in our study with D. menziesii. We thereby propose that the limit of the UST is a useful tool for the determination of the endemic status of Antarctic macroalgal species.
Previous studies suggested that the northern distribution limit of Antarctic endemic Desmarestiales is set by the temperature requirements for sporophyte growth and that sporophytes might represent the bottleneck for fulfillment of the life cycle (Wiencke and tom Dieck 1989; Wiencke et al. 2006, 2007). This does not hold true anymore for D. menziesii, as our results rather suggest that the northern limit of D. menziesii is set by the temperature requirements for the successful reproduction of gametophytes and thereby sporophyte formation at temperatures < 4 °C. This temperature threshold namely conforms to the local SST at the northern distribution limit, at South Georgia Islands.
Conclusion
Climate change and the loss of biodiversity are the two major global threats, and they are not independent from each other. Rising seawater temperatures could lead to a loss of biodiversity in Antarctica, especially threatening the many endemic species. If these stenothermic species are living close to their tolerance limits, they are vulnerable and could be negatively affected under warming (Harley et al. 2006). Climate change is ongoing and most pronounced in Polar Regions (Clarke et al. 2006; IPCC 2019); largest increases in seawater temperature have been recorded in South Georgia Islands (Hogg et al. 2011). As this is the current northern boundary of D. menziesii, especially a temperature increase during winter to spring, during its reproductive season, will probably result in its displacement and consequently a narrowing of its distribution zone.
References
Amsler CD, Rowley RJ, Laur DR, Quetin LB, Ross RM (1995) Vertical distribution of Antarctic Peninsular macroalgae: cover, biomass and species composition. Phycologia 34:424–430. https://doi.org/10.2216/i0031-8884-34-5-424.1
Anderson RJ, Bolton JJ (1989) Growth and fertility, in relation to temperature and photoperiod, in South African Desmarestia firma (Phaeophyceae). Bot Mar 32:149–158
Arkhipkin AI, Grzebielec R, Sirota AM, Remeslo AV, Polishchuk IA, Middleton DAJ (2004) The influence of seasonal environmental changes on ontogenetic migrations of the squid Loligo gahi on the Falkland shelf. Fish Oceanogr 13:1–9
Balestrini C, Manzella G, Lovrich G (1998) Simulación de Corrientes en el Canal Beagle y Bahia Ushuaia, Mediante un Modelo Bidimensional. Servicio De Hidrografía Naval 98:1–58
Barnes DK, Fuentes V, Clarke A, Schloss IR, Wallace MI (2006) Spatial and temporal variation in shallow seawater temperatures around Antarctica. Deep Sea Res Part II 53:853–865
Billard E, Reyes J, Mansilla A, Faugeron S, Guillemin ML (2015) Deep genetic divergence between austral populations of the red alga Gigartina skottsbergii reveals a cryptic species endemic to the Antarctic continent. Polar Biol 38:2021–2034. https://doi.org/10.1007/s00300-015-1762-4
Brawley SH, Johnson LE (1992) Gametogenesis, gametes and zygotes: an ecological perspective on sexual reproduction in the algae. Brit Phycol J 27:233–252. https://doi.org/10.1080/00071619200650241
Carney LT, Edwards MS (2010) Role of nutrient fluctuations and delayed development in gametophyte reproduction by Macrocystis pyrifera (Phaeophyceae) in Southern California. J Phycol 46:987–996
Choi HG, Kim YS, Lee SJ, Park EJ, Nam KW (2005) Effects of daylength, irradiance and settlement density on the growth and reproduction of Undaria pinnatifida gametophytes. J App Phycol 17:423–430. https://doi.org/10.1007/s10811-005-0432-2
Clarke A, Murphy EJ, Meredith MP, King JC, Peck LS, Barnes DK, Smith RC (2006) Climate change and the marine ecosystem of the western Antarctic Peninsula. Philos Trans R Soc B 362:149–166. https://doi.org/10.1098/rstb.2006.1958
Clayton MN (1994) Evolution of the Antarctic marine benthic algal flora. J Phycol 30:897–904
Davenport J, Pugh PJA, McKechnie J (1996) Mixed fractals and anisotropy in subantarctic marine macroalgae from South Georgia: implications for epifaunal biomass and abundance. Mar Ecol Prog Ser 136:245–255. https://doi.org/10.1111/j.0022-3646.1994.00897.x
Dayton PK, Currie V, Gerrodette T, Keller BD, Rosenthal R, Tresca DV (1984) Patch dynamics and stability of some California kelp communities. Ecol Monogr 54:253–289
Ebbing A, Pierik R, Bouma T, Kromkamp JC, Timmermans K (2020) How light and biomass density influence the reproduction of delayed Saccharina latissima gametophytes (Phaeophyceae). J Phycol 56:709–718. https://doi.org/10.1111/jpy.12976
Floc’h JY, Pajot R, Wallentinus I, (1991) The Japanese brown alga Undaria pinnatifida on the coast of France and its possible establishment in European waters. ICES J Mar Sci 47:379–390
Gómez I, Wiencke C (1996) Photosynthesis, dark respiration and pigment contents of gametophytes and sporophytes of the Antarctic brown algae Desmarestia menziesii. Bot Mar 39:149–157
Graiff A, Liesner D, Karsten U, Bartsch I (2015) Temperature tolerance of western Baltic Sea Fucus vesiculosus—growth, photosynthesis and survival. J Exp Mar Biol Ecol 471:8–16
Haderspeck W, Hoffmann KH (1990) Effects of photoperiod and temperature on development and reproduction of Hydromedion sparsutum (Müller) (Coleoptera, Perimylopidae) from South Georgia (Subantarctic). Oecologia 83:99–104
Harley CD, Randall Hughes A, Hultgren KM, Miner BG, Sorte CJ, Thornber CS, Rodriguez LF, Tomanek L, Williams SL (2006) The impacts of climate change in coastal marine systems. Ecol Lett 9:228–241
Hogg OT, Barnes DK, Griffiths HJ (2011) Highly diverse, poorly studied and uniquely threatened by climate change: an assessment of marine biodiversity on South Georgia’s continental shelf. PLoS ONE 6:e19795. https://doi.org/10.1371/journal.pone.0019795
Hommersand MH, Moe RL, Amsler CD, Fredericq S (2009) Notes on the systematics and biogeographical relationships of Antarctic and sub-Antarctic Rhodophyta with descriptions of four new genera and five new species. Bot Mar 52:509–534. https://doi.org/10.1515/BOT.2009.081
Hsiao SIC, Druehl LD (1971) Environmental control of gametogenesis in Laminaria saccharina. I. The effects of light and culture media. Can J Bot 49:1503–1508
Hughey JR, Leister GL, Gabrielson PW, Hommersand MH (2020) Sarcopeltis gen. nov. (Gigartinaceae, Rhodophyta), with S. skottsbergii comb. nov. from southern South America and S. antarctica sp. nov. from the Antarctic Peninsula. Phytotaxa 468:75–88
IPCC (Intergovernmental Panel on Climate Change) (2019) Special report on the ocean and cryosphere in a changing climate. https://www.ipcc.ch/srocc/download-report/
John DM, Tittley I, Lawson GW, Pugh PJA (1994) Distribution of seaweed floras in the Southern Ocean. Bot Mar 37:235–240. https://doi.org/10.1515/botm.1994.37.3.235
Kain JM (1989) The seasons in the subtidal. Br Phycol J 24:203–215
Knox GA (1968) Tides and intertidal zones. Symposium on Antarctic oceanography. Scott Polar Research Institute, Cambridge, pp 131–146
Knox GA (1970) Antarctic marine ecosystems. In: Holdgate MW (ed) Antarctic ecology. Academic Press, New York, pp 65–96
Küpper FC, Peters AF, Shewring DM, Sayer MD, Mystikou A, Brown H, Azzopardi E, Dargent O, Srittmatter M, Brennan D, Asensi O, van West P, Wilce RT (2016) Arctic marine phytobenthos of northern Baffin Island. J Phycol 52:532–549
Küpper FC, Amsler CD, Morley S, de Reviers B, Reichardt A, Peck LS, Peters AF (2019) Juvenile morphology of the large Antarctic canopy-forming brown alga Desmarestia menziesii. J Agardh Pol Biol 42:2097–2103. https://doi.org/10.1007/s00300-019-02584-3
Liesner D, Shama LN, Diehl N, Valentin K, Bartsch I (2020) Thermal plasticity of the kelp Laminaria digitata (Phaeophyceae) across life cycle stages reveals the importance of cold seasons for marine forests. Front Mar Sci 7:456. https://doi.org/10.3389/fmars.2020.00456
Littell RC, Pendergast J, Natarajan R (2005) Tutorial in biostatistics: modelling covariance structure in the analysis of repeated measures data. Stat Med 19:1793–1819
Lüning K (1994) Circadian growth rhythm in juvenile sporophytes of Laminariales (Phaeophyta). J Phycol 30:193–199
Lüning K, Dring MJ (1975) Reproduction, growth and photosynthesis of gametophytes of Laminaria saccharina grown in blue and red light. Mar Biol 29:195–200
Martins N, Pearson GA, Gouveia L, Tavares AI, Serrão EA, Bartsch I (2019) Hybrid vigour for thermal tolerance in hybrids between the allopatric kelps Laminaria digitata and L. pallida (Laminariales, Phaeophyceae) with contrasting thermal affinities. Eur J Phycol 54:548–561
Navarro NP, Huovinen P, Gómez I (2016) Stress tolerance of Antarctic macroalgae in the early life stages. Rev Chil Hist Nat 89:1–9
Navarro N, Huovinen P, Gómez I (2020) Life history strategies, photosynthesis, and stress tolerance in propagules of Antarctic seaweeds. In: Gómez I, Huovinen P (eds) Antarctic seaweeds. Springer, Cham, pp 193–215
Ocaranza-Barrera P, González-Wevar CA, Guillemin ML, Rosenfeld S, Mansilla A (2019) Molecular divergence between Iridaea cordata (Turner) Bory de Saint-Vincent from the Antarctic Peninsula and the Magellan Region. J App Phycol 31:939–949. https://doi.org/10.1007/s10811-018-1656-2
Olabarria C, Arenas F, Viejo RM, Gestoso I, Vaz-Pinto F, Incera M, Rubal M, Cacabelos E, Veiga P, Sobrino C (2013) Response of macroalgal assemblages from rockpools to climate change: effects of persistent increase in temperature and CO2. Oikos 122:1065–1079
Oliveira MC, Pellizzari F, Medeiros AS, Yokoya NS (2020) Diversity of Antarctic seaweeds. In: Gómez I, Huovinen P (eds) Antarctic seaweeds. Springer, Cham, pp 23–42
Papenfuss GF (1964) Catalogue and bibliography of Antarctic and sub-Antarctic benthic marine algae. Am Geophys Union Antarct Res Ser 1:1–76
Peters AF, Breeman AM (1993) Temperature tolerance and latitudinal range of brown algae from temperate Pacific South America. Mar Biol 115:143–150
Pinheiro JC, Bates DM (2000) Linear mixed-effects models: basic concepts and examples. In: Mixed-effects models in S and S-Plus. Springer, London
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2015) nlme: linear and nonlinear mixed effects models. R package version 3.1-120. R Package Version, 3-1
Provasoli L (1968) Media and prospects for the cultivation of marine algae. In: Watanabe A, Hattori A (eds) Cultures and collections of algae. Japan Society of Plant Physiology, Hakone, pp 63–75
Quartino ML, Boraso de Zaixso AL (2008) Summer macroalgal biomass in Potter Cove, South Shetland Islands, Antarctica: its production and flux to the ecosystem. Polar Biol 31:281–294. https://doi.org/10.1007/s00300-007-0356-1
Quartino ML, Zaixso HE, Boraso de Zaixso AL (2005) Biological and environmental characterization of marine macroalgal assemblages in Potter Cove, South Shetland Islands, Antarctica. Bot Mar 48:187–197. https://doi.org/10.1515/BOT.2005.029
Quartino ML, Deregibus D, Campana GL, Latorre GEJ, Momo FR (2013) Evidence of macroalgal colonization on newly ice-free areas following glacial retreat in Potter Cove (South Shetland Islands), Antarctica. PLoS ONE 8:e58223. https://doi.org/10.1371/journal.pone.0058223
Quartino ML, Saravia LA, Campana GL, Deregibus D, Matula CV, Boraso AL, Momo FR (2020) Production and biomass of seaweeds in newly ice-free areas: implications for coastal processes in a changing Antarctic environment. In: Gómez I, Huovinen P (eds) Antarctic seaweeds. Springer, Cham, pp 155–171
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
R Development Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ramirez ME (2010) Flora marina bentónica de la región austral de Sudamérica y la Antártica. An Inst Patagonia 38:57–71. https://doi.org/10.4067/S0718-686X2010000100003
Ramirez ME, Peters AF (1992) The South American species of Desmarestia (Phaeophyceae). Can J Bot 70:2430–2445
Ramirez ME, Müller DG, Peters AF (1986) Life history and taxonomy of two populations of ligulate Desmarestia (Phaeophyceae) from Chile. Can J Bot 64:2948–2954
Rautenberger R, Huovinen P, Gómez I (2015) Effects of increased seawater temperature on UV tolerance of Antarctic marine macroalgae. Mar Biol 162:1087–1097. https://doi.org/10.1007/s00227-015-2651-7
Reed DC (1990) The effects of variable settlement and early competition on pattern of kelp recruitment. Ecology 71:776–787
Reed DC, Neushul M, Ebeling AW (1991) Role of settlement density on gametophyte growth and reproduction in the kelps Pterygophora californica and Macrocystis pyrifera (Phaeophyceae). J Phycol 27:361–366
Robuchon M, Couceiro L, Peters AF, Destombe C, Valero M (2014) Examining the bank of microscopic stages in kelps using culturing and barcoding. Eur J Phycol 49:128–133. https://doi.org/10.1080/09670262.2014.892635
Russell L (2020) emmeans: estimated marginal means, aka least-squares means. R Package Version 1.4.7. https://CRAN.R-project.org/package=emmeans
Savaglia V, Matula CV, Quartino ML, Francione MV, Zacher K (2019) Physiological response to irradiance, temperature and co-cultivation in Antarctic engineering brown algae (Desmarestia menziesii and D. anceps). Polar Biol 42:2031–2044. https://doi.org/10.1007/s00300-019-02578-1
Scher HD, Martin EE (2006) Timing and climatic consequences of the opening of Drake passage. Science 312:428–430. https://doi.org/10.1126/science.1120044
Schoenrock KM, Schram JB, Amsler CD, McClintock JB, Angus RA (2015) Climate change impacts on overstory Desmarestia spp. from the western Antarctic Peninsula. Mar Biol 162:377–389. https://doi.org/10.1007/s00227-014-2582-8
Tan IH, Druehl LD (1996) A ribosomal DNA phylogeny supports the close evolutionary relationships among the Sporochnales, Desmarestiales, and Laminariales (Phaeophyceae). J Phycol 32:112–118
tom Dieck I (1989) Vergleichende Untersuchungen zur Ökophysiologie und Kreuzbarkeit innerhalb der digitaten Sektion der Gattung Laminaria Lamouroux (Phaeophyceae). PhD Thesis, University of Hamburg, Hamburg
tom Dieck I (1992) North Pacific and North Atlantic digitate Laminaria species (Phaeophyta): hybridization experiments and temperature responses. Phycologia 31:147–163
Wells E, Brewin P, Brickle P (2011) Intertidal and Subtidal Benthic Seaweed diversity of South Georgia. Wells Marine, Norfolk
Wiencke C (1990) Seasonality of brown macroalgae from Antarctica: a long-term culture study under fluctuating Antarctic daylengths. Polar Biol 10:589–600
Wiencke C, Amsler CD (2012) Seaweeds and their communities in Polar Regions. In: Wiencke C, Bischof K (eds) Seaweed biology, novel insights into ecophysiology, ecology and utilization ecological studies, vol 219. Springer, Heidelberg, pp 265–292. https://doi.org/10.1007/978-3-642-28451-9
Wiencke C, Clayton M (2002) Antarctic seaweeds. In: Wägele JW (ed) Synopsis of the Antarctic Benthos. ARG Gantner, Ruggell, Liechtenstein
Wiencke C, tom Dieck I (1990) Temperature requirements for growth and survival of macroalgae from Antarctica and southern Chile. Mar Ecol Prog Ser 599:157–170
Wiencke C, tom Dieck I (1989) Temperature requirements for growth and temperature tolerance of macroalgae endemic to the Antarctic region. Mar Ecol Prog Ser 54:189–197
Wiencke C, Bartsch I, Bischoff B, Peters AF, Breeman AM (1994) Temperature requirements and biogeography of Antarctic, Arctic and amphiequatorial seaweeds. Bot Mar 37:247–260
Wiencke C, Clayton MN, Schulz D (1995) Life history, reproductive morphology and development of the Antarctic brown alga Desmarestia menziesii. J Agardh Bot Acta 108:201–208
Wiencke C, Roleda MY, Gruber A, Clayton MN, Bischof K (2006) Susceptibility of zoospores to UVR determines upper depth distribution limit of Arctic kelps: evidence through field experiments. J Ecol 94:455–463. https://doi.org/10.1111/j.1365-2745.2006.01102.x
Wiencke C, Clayton MN, Gómez I, Iken K, Lüder UH, Amsler CD, Karsten U, Hanelt D, Bischof K, Dunton K (2007) Life strategy, ecophysiology and ecology of seaweeds in polar waters. Rev Environ Sci Bio/technol 6:95–126. https://doi.org/10.1007/s11157-006-9106-z
Wiencke C, Amsler CD, Clayton MN (2014) Macroalgae. In: De Broyer C, Koubbi P, Griffiths HJ, Raymond B, Udekem d’Acoz, C d’,Van de Putte AP, Danis B, David B, Grant S, Gutt J, Held C, Hosie G. Huettmann F, Post A, Ropert-Coudert Y (eds) Biogeographic atlas of the Southern Ocean. Scientific Committee on the Antarctic Research, Cambridge, pp 66–73
Zacher K, Rautenberger R, Hanelt D, Wulff A, Wiencke C (2009) The abiotic environment of polar marine benthic algae. Bot Mar 52:483–490. https://doi.org/10.1515/BOT.2009.082
Zacher K, Savaglia V, Bartsch I (2016) Effects of temperature and interspecific competition on growth and photosynthesis of two endemic Antarctic Desmarestia species. Algol Stud 151:103–122. https://doi.org/10.1127/algol_stud/2016/0269
Zuur A, Ieno EN, Smith GM (2007) Analysing ecological data. Springer, New York
Acknowledgements
This study was performed with the financial support of Instituto Antártico Argentino/Dirección Nacional del Antártico, ANPCyT-DNA (PICT 2017-2691), the EU project Research Network IMCONet funded by the Marie Curie Action IRSES IMCONet (FP7 IRSES, Action No. 318718), the cooperation project between Argentina and Germany, MINCYT – BMBF 2017 (SEACOAST – AL/17/06), and EU 872690 (Horizon 2020-Marie Skłodowska-Curie Action RISE – 2019 – Research and Innovation Staff Exchange) “Coastal ecosystem carbon balance in times of rapid glacier melt” (CoastCarb). This work was further supported by the Deutsche Forschungsgemeinschaft (DFG) in the framework of the priority programme ‘Antarctic Research with comparative investigations in Arctic ice areas’ by a grant Za735/1-1. We thank Andreas Wagner and Claudia Daniel for their technical support in the laboratory.
Author information
Authors and Affiliations
Contributions
The experiments were planned and designed by KZ, CVM, and IB. CVM carried out the experiments in the AWI laboratories. CVM and JDN performed the statistical analyses. KZ and IB supervised the project and all authors contributed to data interpretation and discussion. CMV wrote the manuscript, which was reviewed and revised by all authors. All authors gave their approval of the final version for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Matula, C.V., Quartino, M.L., Nuñez, J.D. et al. Effects of seawater temperature and seasonal irradiance on growth, reproduction, and survival of the endemic Antarctic brown alga Desmarestia menziesii (Phaeophyceae). Polar Biol 45, 559–572 (2022). https://doi.org/10.1007/s00300-021-02991-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-021-02991-5