Abstract
Seabirds show a range of patterns of sexual size dimorphism and sex-specific parental investment, but the underlying causes remain poorly understood. The aim of the present study was to test two longstanding hypotheses of parental investment in a sexually monomorphic species, Wilson’s storm petrel Oceanites oceanicus, namely that males attend chicks more frequently and females deliver larger meals (Beck and Brown in Br Antarct Surv Sci Rep 69:1–54, 1972). We recorded in eight seasons, both during incubation and chick rearing, which adult was caught first in a nest and found no difference in the probability of catching a male or a female first in any year. Additionally, in five seasons we employed a miniature video camera to record nest attendance during chick rearing and found no significant difference except for 2006, a year with very low krill availability, where females visited the nest less often than males. We then combined video observations with periodic weighing of chicks to estimate mean daily feeding mass (g/day) of males and females and found no difference in the amount of food delivered per day between the sexes. However, in years with low krill availability, males and females tended to use different strategies to achieve the same feeding rates, with females undertaking longer foraging trips and delivering heavier meals. Thus, our results do not support the hypothesis of a general sex-specific parental investment in Wilson’s storm petrels, but a tendency for a context-dependent sex-specific investment in the years of food shortage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biparental care, where males and females participate in chick feeding, is known in 75–90% of all bird species (Cockburn 2006; Lack 1968). In seabirds, which rely on temporally and spatially unpredictable food resources in their marine environment, provisioning by both parents is often necessary to guarantee successful chick rearing, but males and females may differ in their investment (Bradley et al. 2002; Creelman and Storey 1991; Harding et al. 2004). Several studies have found evidence for differences between the sex in food provisioning and foraging behaviour (Gonzalez-Solis et al. 2000; Lewis et al. 2002, 2005; Quillfeldt et al. 2004). Sexual size dimorphism was one of the first reasons that was thought to lead to differential parental investment, with either the larger sex dominating intraspecific interactions close to the colony that force the smaller sex to fly longer distances (Gonzalez-Solis et al. 2000) or the smaller sex having a better flight efficiency enabling it to travel to more profitable feeding grounds (Shaffer et al. 2001). Information on sex-specific parental investment for monomorphic seabird species is still relatively scarce, mainly due to the difficulties in discriminating between sexes in the field. However, there are recent results, which indicate that sex-specific parental investment also occurs in monomorphic seabird species (Gray and Hamer 2001; Paredes et al. 2006; Peck and Congdon 2006; Thaxter et al. 2009). In Manx shearwaters Puffinus puffinus and Wedge-tailed shearwaters P. pacificus, males were found to invest more in chick feeding (Gray and Hamer 2001; Peck and Congdon 2006), whereas in Atlantic puffins Fratercula arctica, females invest more in the direct care of offspring and males more in nest defence (Creelman and Storey 1991). Discussed as underlying causes are differences in the condition at the start of the chick-rearing period (Gray and Hamer 2001; Lewis et al. 2002), the nest attendance times (Woo et al. 1999), the sensitivity to the needs of the chick (Hamer et al. 2006; Quillfeldt and Masello 2004) and foraging efficiency (Gray and Hamer 2001; Lewis et al. 2002).
Here, we study sex-specific parental investment in Wilson’s storm petrels Oceanites oceanicus, a small procellariiform seabird with a negligible sexual size dimorphism, with females being about 2% larger than males (Büßer 2003: wing: 2.8%, tarsus: 1.7%, tail: 2.2%) and 4% heavier (e.g. Quillfeldt et al. 2006). Wilson’s storm petrels are the smallest endotherm animals breeding in the Antarctic and descriptions of their basic biology and life cycle can be found in Roberts (1940) and Beck and Brown (1972). They lay single-egg clutches, starting in mid-December. Hatching mainly takes place in the first half of February and chicks stay in the nest burrows for about 60 days. During the day, they are left unattended and the adults feed them during nocturnal visits until fledging. Fledging starts in the second half of March. Breeding success is highly variable between years (0–59%); failures are caused by entombment due to snowstorms and food shortage (Büßer et al. 2004, 2008). Wilson’s storm petrels have a monogamous mating system (Quillfeldt et al. 2001) and intense parental care is shared between sexes during incubation and chick rearing to a so far unknown extent. Beck and Brown (1972) suggested a sex difference in parental investment during their study on Wilson’s storm petrels on Signy Island, South Orkney Islands and reported that males visited the nest burrows more frequently, whereas females delivered greater meals. This pattern may be related to a continuation of the behaviour between copulation and egg laying, where the female departs the colony for about 10 days to feed intensely in krill-rich waters to form a large egg (“pre-laying exodus”), while the male visits the nest burrow to keep the entrance snow-free and accessible to the female when she returns (Beck and Brown 1972). However, Beck and Brown (1972) were not able to test this statement with figures of the amount of food brought to the nest by the parents, as the methods of estimation of meal sizes from periodic weighing of chicks were not developed until a decade later (Ricklefs et al. 1985).
In this study, we therefore tested the following hypotheses:
-
1.
Males visit the nest burrow more frequently. In this case, the probability of catching a male first at a nest is significantly higher than 50%, and male visits are recorded significantly more often on video observations.
-
2.
Females deliver heavier meals to the nest than males.
Materials and methods
Field work took place from January to March of the years 1996, 1998, 1999–2003, 2005 and 2006, at a colony of 1,400 and 2,200 breeding pairs of Wilson’s storm petrels (Hahn et al. 1998) on King George Island, South Shetland Island (62°14′S, 58°40′W) in the maritime Antarctic.
To examine the nest attendance of males and females during incubation and chick rearing, we recorded the sex of the first captured adult per nest in each season (n = 868 nests, 42–159 per season). If males and females attend the nest equally, the probability of recording an adult of a certain sex as the first captured adult should not be significantly different from 50%. Nests were marked in the beginning of the 1996 breeding season, and further nests were marked in the following seasons. Marked nests were monitored for eggs and hatching chicks. If an adult was attending the nest, it was taken out of the nest burrow by hand. Adults were captured in the nest only once, either during incubation or chick feeding and released back to their nest burrow afterwards. Captured adults where ringed and weighed with a digital 100 g balance to the nearest 0.1 g using a weighing cone. A blood sample for molecular sexing (approximately 50 μl) was taken by puncture of the brachial vein (license of the Environmental Agency (Umwelt-Bundesamt) of Germany). Molecular gender determination was carried out through PCR after extracting DNA from the blood samples (Griffiths et al. 1996; Lubjuhn and Sauer 1999).
In order to assess nest visits by both sexes in the seasons 2000–2002, 2005 and 2006, we additionally monitored nest burrows during the night with miniature infrared cameras that recorded both visual and acoustic information (Masello et al. 2001). The cameras were placed inside the nest and connected to a video recording system that enabled us to identify the feeding adult and to record the frequency and time of nest visits. A total of 35 nests were filmed, 2 in 2000, 2 in 2001, 3 in 2002, 16 in 2005 and 12 in 2006, covering between 1 and 22 nights per nest. Filmed nests differed between the years. To discriminate between the adults on the videotapes, we marked one adult of each breeding pair with a dot of silver acrylic paint on the forehead and on the bow of the wing. We started video recording each day at sunset and collected videotapes in the morning. On the basis of a total of 720 h of film material, the duration of stay, the number of food transfers to the chick (regurgitations) and the duration of feeding events were recorded. In the case of several feeding events by the same adult during one night, these events were pooled as one feeding as they resulted from one foraging trip. We estimated meal sizes from daily mass differences of chicks corrected for metabolic mass loss (Quillfeldt and Peter 2000; Ricklefs et al. 1985). Meal sizes were defined as one feeding event for net mass increments up to 13 g and two for increments larger than 13 g (Obst and Nagy 1993; Quillfeldt and Peter 2000). As we have several measurements of meal size per adult, we summarised these before the analysis to avoid pseudoreplications. Thus, for each adult, a mean value was used in statistical analysis.
Statistical analysis
Statistical tests were performed in SPSS 11.0. Normality was tested with Kolmogorov–Smirnov tests. Means are given with standard errors. We assessed significance using F statistics in general linear models (GLM), based on type III sum of squares if the assumptions of normality and equal variance were met. Χ 2 tests were used to test adult nest attendance according to capture probability. The significance level was set to P < 0.05. Because the mean values of meal sizes calculated for every individual were based on different sample sizes, we used weighted least squares models for our statistical analyses. To estimate the effects of year and sex, we used successive difference contrasts implemented in the MASS package (Venables and Ripley 2002) in R 2.8.1 (R Development Core Team 2008, www.r-project.org). Weighted least squares models minimise the weighted sum of the squared residuals, sum (w*e2), where e is the vector of residuals and w is the vector of sample sizes. For nest attendance recorded on video, the assumption of equal variance was not met; we therefore used Kruskall–Wallis tests for the single years. As in this case, several tests of a single null hypothesis were carried out. We added alpha-level adjustments as follows. We corrected significant P values for the number of tests, applying the following equation: P corr = 1 – (1 – α′)k, which we derived from conversion of the Dunn-Šidák method (Sokal and Rohlf 1995). In this equation, P corr denotes the corrected P value, α′ equals the originally derived P value, and k equals the number of tests. Note that sample sizes may vary between analyses, as not all variables could be recorded for each nest.
Results
Adult nest attendance according to capture probability
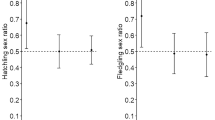
The probability of catching a male first in a nest did not deviate significantly from 50% in any 1 year (Fig. 1; Χ 2 for single years: all P values >0.05; Χ 2 for incubation period in all years: Χ 2 = 7.2, df = 8, P = 0.52; Χ 2 for chick rearing period in all years: Χ 2 = 8.4; df = 8, P = 0.40). Thus, neither during the incubation nor the chick-rearing period males visited the nest significantly more often than females.
Adult nest attendance and feeding behaviour from video data
In 70 nights, both adults visited the nest, while 90 feedings by a single adult were observed (of which 83 could be assigned to male or female). In all but one case, all nest visits were associated with chick feeding, such that the frequency of nest visits equaled the feeding frequency. Males and females attended the nest on 75 and 66% (Table 1) of all recorded nights, respectively, including the 70 nights in which both adults visited the nest. We found that in 1 year, 2006, females visited the nest burrows less frequently than males (Table 1; Kruskall–Wallis, df = 1, P = 0.011, P corr = 0.05), although this difference did not appear in any other year (Table 1; Fig. 2, Kruskall–Wallis, all P > 0.1). Overall, adults fed the chick an average of 9.63 ± 1.25 min per night (n = 162). The mean meal size of observed single feedings showed no difference between the sexes in 2000, 2001, 2005 and 2006 (Table 1; Fig. 2, GLM, year: F 4,26 = 5.30, P = 0.003; sex: F 1,26 = 12.57, P = 0.002; year*sex: F 4,26 = 2.96, P = 0.038), but was significantly larger for females in 2002 (Table 1; Fig. 2, Tukey test: P = 0.003).
Comparison of male (black) and female (white) Wilson’s storm petrels (a) Frequency of nest attendance by pair members inferred from video observations of nest burrows on King George Island. The frequency of nest attendance in this study was a good proxy for the feeding frequency. Note that in 2001, nest attendance was equal for both sexes and only one point appears in the figure. b Mean meal size determined for single feedings. c Mean daily feeding mass. Means are given with standard errors
The mean daily feeding mass represents the product of nest attendance and mean single meal size per sex and nest and may be used as a measurement of parental investment. The mean daily feeding mass did not differ between the sexes in any year (Table 1; Fig. 2, GLM, year: F 4,33 = 0.69, P = 0.60; sex: F 1,33 = 0.00, P = 0.99; year*sex: F 4,33 = 0.10, P = 0.98). The fact that females delivered larger meals in 2006 and visited the nest burrow less frequently in 2002 was counterbalanced by the tendency to less frequent feedings and bigger meals, respectively (Fig. 2). In most cases, adults stayed in the nest burrow after feeding the chick, the duration of the stay ranging from 6 to 396 min with a mean of 113.18 ± 9.69 min.
Discussion
In our study, we found no general evidence for sex-specific parental investment in Wilson’s storm petrel, but a tendency to a context-dependent investment with males and females using different ways of achieving the same amount of investment in the years of food shortage. As a result, the mean daily feeding mass to the chick did not differ significantly between males and females in any year.
We found no general support of the pattern described in Beck and Brown (1972) that males visit the nest more frequently and females deliver meals of a larger size in Wilson’s storm petrels. The probability to catch an adult of a certain sex first was not significantly sex-biased in any year. In 2002 and 2006, we found a slight tendency for males to visit the nest more frequently, bringing small amounts of food, whereas females seemed to undertake longer foraging trips resulting in larger meals. In these 2 years, a very low krill abundance was observed in the Elephant Island Region (Büßer et al. 2004; Reiss et al. 2008), the estimated foraging range of the Wilson’s storm petrels breeding at the study colony on King George Island (Gladbach et al. 2007; Pennycuick et al. 1984). The krill index calculated for 2000–2002 in Büßer et al. (2004) shows a high correlation with female nest attendance and female mean meal size (Krill index, female nest attendance: positive, R = 0.82 and Krill index, female mean meal size: negative, R = 0.99). However, the overall investment per chick measured as the mean daily feeding mass did not show a difference between the sexes, and the contribution to chick rearing was shared equally. Especially in years of low food abundance, males and females may use different strategies to achieve the same total amount of investment.
This is in line with our expectations based on the lifestyle of Wilson’s storm petrels. Their dependence on spatially and temporally variable resources requires the investment of both pair members to assure the successful rearing of a chick. During the study period, Wilson’s storm petrels at King George Island had very low overall breeding success and frequent failures (Büßer et al. 2004). In particular, total breeding failures of the colonies due to entombment of chicks after snowstorms distinguish them from many other long-lived Procellariiformes, and both adults in Wilson’s storm petrels may thus be required to invest heavily in each chick to compensate for this disadvantage. Furthermore, they are socially and genetically monogamous (Quillfeldt et al. 2001), so that parental investment is not affected by an uncertainty about paternity, which may cause a sex bias in caring (Kokko and Jennions 2003).
We propose two possible scenarios to explain the occurrence of a sex difference in provisioning in years of low food availability.
-
(1)
Competition in foraging grounds: sex-specific foraging despite being monomorphic could occur if one sex is more efficient at foraging (Lewis et al. 2002; Peck and Congdon 2006; Thaxter et al. 2009), leading to a niche divergence as a way of reducing competition between the sexes (Gonzalez-Solis et al. 2000; Gray and Hamer 2001; Thaxter et al. 2009). In years with high krill abundance, the difference in foraging efficiency between males and females may be negligible and both pair members use the same foraging grounds. However, in years of food shortage, females may avoid this competition by undertaking longer foraging trips to more distant foraging grounds.
-
(2)
Responsiveness to solicitation: novel results relate the differences in chick provisioning to a different responsiveness of males and females to signals of chick state (Quillfeldt et al. 2004). Already described in several passeriform birds (reviewed in Kilner 2002), this pattern could also be found in seabird species (Quillfeldt et al. 2004). In Manx shearwaters, where males make a greater contribution to overall provisioning, females adjusted their foraging effort to the begging intensity and body condition of their chick, but males did not (Quillfeldt et al. 2004). For an extensive discussion of the possible explanations, see Quillfeldt et al. (2004). This may explain the pattern we found for years with lower food availability, where females could have responded to the needs of the chick by undertaking longer foraging trips. That chicks of Wilson’s storm petrels indicate their needs via begging and that parents are responsive to this solicitation behaviour has been shown previously (Gladbach et al. 2009; Quillfeldt 2002).
Our results may explain why studies of sex-specific investment in monomorphic seabird species yield equivocal results (differences in investment: Gray and Hamer 2001; Paredes et al. 2006; Peck and Congdon 2006; Thaxter et al. 2009; no differences: Quillfeldt et al. 2007). Differences in provisioning may depend on the amount of food supplies available around a certain study colony, leading to the result of equal provisioning patterns in study colonies with a good supply or in a year with high food availability, but sex differences in provisioning for colonies in short supply or a year of low prey density in the foraging grounds. Furthermore, both meal size and frequency of nest visits should be taken into account when investigating the sex-specific amount of investment, as a variation in one of these variables may be counterbalanced by the other. Long-term studies combining the observation of the sex-specific amount of investment with the monitoring of prey availability in different years are necessary to answer the question of sex-specific parental investment in monomorphic seabird taxa.
References
Beck JR, Brown DW (1972) The biology of Wilson’s storm petrel, Oceanites oceanicus (Kuhl), at Signy Island, South Orkney Islands. Br Antarct Surv Sci Rep 69:1–54
Bradley RW, McFarlane LA, Tranquilla LAM, Vanderkist BA, Cooke F (2002) Sex differences in nest visitation by chick rearing marbled Murrelets. Condor 104:178–183. doi:10.1650/0010-5422(2002)104[0178:SDINVB]2.0.CO;2
Büßer C (2003) Elterliche Investition und Morphometrie der Buntfußsturmschwalbe, Oceanites oceanicus, auf King George Island, Südshetland-Inseln, Antarktis (Diploma thesis), Friedrich-Schiller-University, Jena
Büßer C, Kahles A, Quillfeldt P (2004) Breeding success and chick provisioning in Wilson’s storm petrels Oceanites oceanicus over seven years: frequent failures due to food shortage and entombment. Polar Biol 27:613–622
Büßer C, Hahn S, Gladbach A, Lorenz S, Nordt A, Quillfeldt P, Schmoll T, Peter H-U (2008) A decade of fundamental ecological research on storm petrels at the Tres Hermanos colony, Potter Peninsula, King George Island. Rep Pol Res 571:168–175
Cockburn A (2006) Prevalence of different modes of parental care in birds. Proc R Soc Lond Ser B Biol Sci 273:1375–1383
Creelman E, Storey AE (1991) Sex differences in reproductive behavior of Atlantic puffins. Condor 93:390–398. doi:10.2307/1368955
Gladbach A, McGill RAR, Quillfeldt P (2007) Foraging areas of Wilson’s storm petrel Oceanites oceanicus in the breeding and inter-breeding period determined by stable isotope analysis. Polar Biol 30:1005–1012. doi:10.1007/s00300-007-0258-2
Gladbach A, Büßer C, Mundry R, Quillfeldt P (2009) Acoustic parameters of begging calls indicate chick body condition in Wilson’s storm petrel Oceanites oceanicus. J Ethol 27:267–274. doi:10.1007/s10164-008-0115-y
Gonzalez-Solis J, Croxall JP, Wood AG (2000) Sexual dimorphism and sexual segregation in foraging strategies of northern giant petrels, Macronectes halli, during incubation. Oikos 90:390–398. doi:10.1034/j.1600-0706.2000.900220.x
Gray CM, Hamer KC (2001) Food-provisioning behaviour of male and female Manx shearwaters, Puffinus puffinus. Anim Behav 62:117–121. doi:10.1006/anbe.2001.1717
Griffiths R, Daan S, Dijkstra C (1996) Sex identification in birds using two CHD genes. Proc R Soc Lond Ser B Biol Sci 263:1251–1256
Hahn S, Peter HU, Quillfeldt P, Reinhardt K (1998) The birds of the Potter Peninsula, King George Island, South Shetland Islands, Antarctica, 1965–1998. Mar Ornithol 26:1–6
Hamer KC, Quillfeldt P, Masello JF, Fletcher KL (2006) Sex differences in provisioning rules: responses of Manx shearwaters to supplementary chick feeding. Behav Ecol 17:132–137. doi:10.1093/beheco/arj008
Harding AMA, Van Pelt TI, Lifjeld JT, Mehlum F (2004) Sex differences in Little Auk Alle alle parental care: transition from biparental to paternal-only care. Ibis 146:642–651. doi:10.1111/j.1474-919X.2004.00297.x
Kilner RM (2002) Sex differences in canary (Serinus canaria) provisioning rules. Behav Ecol Sociobiol 52:400–407. doi:10.1007/s00265-002-0533-8
Kokko H, Jennions M (2003) It takes two to tango. Trends Ecol Evol 18:103–104. doi:10.1016/S0169-5347(03)00009-0
Lack D (1968) Ecological adaptations for breeding in birds. Methuen & Co., London
Lewis S, Benvenuti S, Dall’Antonia L, Griffiths R, Money L, Sherratt TN, Wanless S, Hamer KC (2002) Sex-specific foraging behaviour in a monomorphic seabird. Proc R Soc Lond Ser B Biol Sci 269:1687–1693
Lewis S, Schreiber EA, Daunt F, Schenk GA, Orr K, Adams A, Wanless S, Hamer KC (2005) Sex-specific foraging behaviour in tropical boobies: does size matter? Ibis 147:408–414. doi:10.1111/j.1474-919x.2005.00428.x
Lubjuhn T, Sauer KP (1999) Fingerprinting and profiling in behavioural ecology. In: Epplen JT, Lubjuhn T (eds) DNA profiling and DNA fingerprinting. Birkhäuser, Basel, pp 39–52
Masello JF, Pagnossin GA, Palleiro GE, Quillfeldt P (2001) Use of miniature security cameras to record behaviour of burrow-nesting birds. Die Vogelwarte Kurze Mitteilungen 41:150–154
Obst BS, Nagy KA (1993) Stomach oil and the energy budget of Wilsons storm petrel nestlings. Condor 95:792–805. doi:10.2307/1369418
Paredes R, Jones IL, Boness DJ (2006) Parental roles of male and female thick-billed murres and razorbills at the Gannet Islands, Labrador. Behaviour 143:451–481. doi:10.1163/156853906776240641
Peck DR, Congdon BC (2006) Sex-specific chick provisioning and diving behaviour in the wedge-tailed shearwater Puffinus pacificus. J Avian Biol 37:245–251. doi:10.1111/j.2006.0908-8857.03558.x
Pennycuick CJ, Croxall JP, Prince PA (1984) Scaling of foraging radius and growth-rate in petrels and Albatrosses (Procellariiformes). Ornis Scand 15:145–154. doi:10.2307/3675955
Quillfeldt P (2002) Begging in the absence of sibling competition in Wilson’s storm petrels, Oceanites oceanicus. Anim Behav 64:579–587. doi:10.1006/anbe.2002.3090
Quillfeldt P, Masello JF (2004) Context-dependent honest begging in Cory’s shearwaters, Calonectris diomedea—influence of food availability. Acta Ethol 7:73–80. doi:10.1007/s10211-004-0100-6
Quillfeldt P, Peter HU (2000) Provisioning and growth in chicks of Wilson’s storm petrels, Oceanites oceanicus, on King George Island, South Shetland Islands. Polar Biol 23:817–824. doi:10.1007/s003000000158
Quillfeldt P, Schmoll T, Peter HU, Epplen JT, Lubjuhn T (2001) Genetic monogamy in Wilson’s storm petrel. Auk 118:242–248. doi:10.1642/0004-8038(2001)118[0242:GMIWSS]2.0.CO;2
Quillfeldt P, Masello JF, Hamer KC (2004) Sex differences in provisioning rules and honest signalling of need in Manx shearwaters, Puffinus puffinus. Anim Behav 68:613–620. doi:10.1016/j.anbehav.2003.12.002
Quillfeldt P, Masello JF, Lubjuhn T (2006) Variation in the adult body mass of Wilson’s storm petrels Oceanites oceanicus during breeding. Polar Biol 29:372–378. doi:10.1007/s00300-005-0066-5
Quillfeldt P, Strange IJ, Segelbacher G, Masello JF (2007) Male and female contributions to provisioning rates of thin-billed prions, Pachyptila belcheri, in the South Atlantic. J Ornithol 148:367–372
Reiss CS, Cossio AM, Loeb V, Demer DA (2008) Variations in the biomass of Antarctic krill (Euphausia superba) around the South Shetland Islands, 1996–2006. ICES J Mar Sci 65:497–508. doi:10.1093/icesjms/fsn033
Ricklefs RE, Day CH, Huntington CE, Williams JB (1985) Variability in feeding rate and meal size of Leach’s storm petrel at Kent-Island, New Brunswick. J Anim Ecol 54:883–898. doi:10.2307/4385
Roberts B (1940) The life cycle of Wilson’s Petrel Oceanites oceanicus (Kuhl). Br Graham Land Exped Sci Rep 1:141–194
Shaffer SA, Weimerskirch H, Costa DP (2001) Functional significance of sexual dimorphism in Wandering Albatrosses, Diomedea exulans. Funct Ecol 15:203–210. doi:10.1046/j.1365-2435.2001.00514.x
Sokal RR, Rohlf FJ (1995) Biometry, 3rd edn. W.H. Freeman & Company, New York
Thaxter CB, Daunt F, Hamer KC, Watanuki Y, Harris MP, Gremillet D, Peters G, Wanless S (2009) Sex-specific food provisioning in a monomorphic seabird, the common guillemot Uria aalge: nest defence, foraging efficiency or parental effort? J Avian Biol 40:75–84. doi:10.1111/j.1600-048X.2008.04507.x
Venables WN, Ripley BD (2002) Modern Applied Statistics with S, 4th edn. Springer, Berlin
Woo K, Kober K, Gaston AJ (1999) Difference in timing of incubation shifts between male and female thick-billed murres are associated with variation in maximum diving depth. Pac Seab 26:55
Acknowledgments
We would like to thank Thomas Lubjuhn for sharing his knowledge and providing lab facilities for the molecular sex determination, and Tim Schmoll, Steffen Hahn and Markus Ritz for their contributions to logistics and field work. We are very grateful to Christoph Scherber for his statistical advice. The manuscript benefited from the comments of the three anonymous referees. We received logistic support from the Alfred-Wegener Institute of Marine and Polar Research (Bremerhaven, Germany), the National Antarctic Institute of Argentina and Hapag Lloyd Seetouristik. This study was partly funded by grants povided by the Deutsche Forschungsgemeinschaft (Pe 454), BMBF-DLR, Studienstiftung des Deutschen Volkes and the State of Thuringia, Germany (Landesgraduiertenstipendium).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Gladbach, A., Braun, C., Nordt, A. et al. Chick provisioning and nest attendance of male and female Wilson’s storm petrels Oceanites oceanicus . Polar Biol 32, 1315–1321 (2009). https://doi.org/10.1007/s00300-009-0628-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-009-0628-z