Abstract
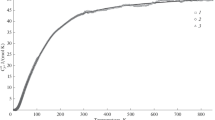
The low-temperature heat capacity of ɛ-Mg2PO4OH was measured between 10 and 400 K by adiabatic calorimetry. No phase transition was observed over this temperature range. A relative enthalpy increment of 22,119 J mol−1 and an absolute entropy value of 127.13±0.25 J mol−1 K−1 at 298.15 K are derived from the results. The low-temperature heat-capacity data are compared with the DSC data obtained from 143 K to 775 K and show marginal differences in the common temperature range. The latter data are fitted by the polynomial
which allows extrapolation to high temperatures.


Similar content being viewed by others
References
Annersten H, Nord AG (1980) A high pressure phase of magnesium orthophosphate. Acta Chem Scand 34:389–390
Benisek A, Dachs E, Cemič L (1999) Heat capacities of Tschermak substituted Fe-biotite. Contrib Mineral Petrol 135:53–61
Berman RG, Brown TH (1985) Heat capacity of minerals in the system Na2O–K2O–CaO–MgO–FeO–Fe2O3–Al2O3–SiO2–TiO2–H2O–CO2: representation, estimation, and high temperature extrapolation. Contrib Mineral Petrol 89:168–183
Berthet G, Joubert JC, Bertaut EF (1972) Vacancies ordering in new metastable orthophosphates (Co3)P2O8 and (Mg3)P2O8 with olivine-related structure. Z Kristallogr 136:98–105
Bolland MDA, Glencross RN, Gilkes RJ, Kumar V (1992) Agronomic effectiveness of partially acidulated rock phosphate and fused calcium-magnesium phosphate compared with superphosphate. Fertil Res 32:169–183
Bosenick A, Geiger CA, Cemič L (1996) Heat capacity measurements of synthetic pyrope-grossular garnets between 320 and 1000 K by differential scanning calorimetry. Geochim Cosmochim Acta 60:3215–3227
Brunet F, Vielzeuf D (1996) The farringtonite / Mg3(PO4)2-II transformation: a new curve for pressure calibration in piston-cylinder apparatus. Eur J Mineral 8:349–354
Brunet F, Chopin C, Seifert F (1998) Phase relations in the MgO–P2O5–H2O system and the stability of phosphoellenbergerite: petrological implications. Contrib Mineral Petrol 131:54–70
Brunet F, Morineau D, Schmid-Beurmann P (2004) Heat-capacity of lazulite, MgAl2(PO4)2(OH)2, from 35 to 298 K and a (S–V) value for P2O5 to estimate phosphates entropy. Mineral Mag 68:123–134
Ditmars DA, Douglas TB (1971) Measurements of the relative enthalpy of pure α-Al2O3 (NBS heat capacity and enthalpy reference material No. 720) from 273 to 1173 K. J Res 75A:401–420
Driessens FCN, Boltong MG, Zapatero MI, Verbeeck RMH, Bonfield W, Bermudez O, Fernandez E, Ginebra MP, Planell JA (1995) In-vivo behavior of 3 calcium-phosphate cements and a magnesium phosphate cement. J Mater Sci - Mater in Medicine 6:272–278
Fasshauer DW, Chatterjee ND, Cemič L (1998) A thermodynamic analysis of the system LiAlSiO4-NaAlSiO4-Al2O3-SiO2-H2O based on new heat capacity, thermal expansion, and compressibility data for selected phases. Contrib Mineral Petrol 133:186–198
Holland TJB (1989) Dependence of entropy on volume for silicate and oxide minerals: A review and a predictive model. Am Mineral 74:5–13
Jaulmes S, Elfakir A, Quarton M, Brunet F, Chopin C (1997) Structure cristalline de la phase haute température et haute pression de Mg3(PO4)2. J Solid State Chem 129:341–345
Leyx C, Chopin C, Brunet F, Schmid-Beurmann P, ParraT (2002) Towards a thermodynamic database for phosphate minerals: volume properties of Mg-phosphates and phase relations in the system MgO–Al2O3–P2O5–SiO2–H2O. IMA 18th general meeting, Edinburgh, Programme with Abstracts, p 240
Lodders K (1999) Revised thermochemical properties of phosphinidene (PH), phosphine (PH3), phosphorus nitride (PN), and magnesium phosphate (Mg3P2O8). J Phys Chem Ref Data 28:1705–1712
Lyon JE, Fox TU, Lyons JW (1966) Phosphate bonding of magnesia refractories. Am Ceram Soc Bull 45:1078–1081
Miltenburg JC van, Genderen ACG van, Berg GJK van den (1998) Design improvements in adiabatic calorimetry. The heat capacity of cholesterol between 10 and 425 K. Thermochim Acta 319:151–162
Miltenburg JC van, Berg GJK van den, Bommel MJ van (1987) Construction of an adiabatic calorimeter. Measurement of the molar heat capacity of synthetic sapphire and of n-heptane. J Chem Thermodyn 19:1129–1137
Oetting FL, McDonald RA (1963) The thermodynamic properties of magnesium orthophosphate and magnesium pyrophosphate. J Phys Chem 67:2737–2743
Preston-Thomas H (1990) The International Temperature Scale of 1990 (ITS-90). Metrologia 27:3–10
Raade G (1990) Hydrothermal syntheses of Mg2PO4OH polymorphs. N Jb Miner Mh 1990:289–300
Raade G, Rømming C (1986) The crystal structure of ɛ-Mg2PO4OH, a synthetic high-temperature polymorph. Zeit Kristallographie 177:1–13
Sarkar AK (1990) Phosphate cement-based fast-setting binders. Am Ceram Soc Bull 69:234–238
Sata T (1997) Phase relations in the system Ca3(PO4)2–CaMg(SiO3)2–MgSiO3–SiO2. J Ceram Soc Japan 105:26–30
Stevens CG, Turkdogan ET (1954) The heats of formation of trimanganous phosphate and trimagnesium phosphate. Trans Faraday Soc 50:370–373
Sutor D, Wooley SE, Ellingsworth JJ (1974) Some aspects of the urinary stones in Great Britain and Northern Ireland. Brit J Urol 46:275–288
Théodoret, Lenzi J, Roux P, Bonel G, Lenzi M (1987) Comportement sous haute pression d’orthophosphates mixtes de calcium et de cobalt Ca3-x Co x (PO4)2, 0,40<x<3. Rev Chim Minér 24:478–488
Yang Q, Zhu B, Wu X (2000) Characteristics and durability tests of magnesium phosphate cement-based material for rapid repair of concrete. Materials and Structures 33:229–234
Acknowledgements
We thank Mrs Kluge, Kiel, for the DSC measurements, Fabrice Brunet and Peter Schmid-Beurmann for numerous discussions, Gunnar Raade and the anonymous referee for their careful reviews.
Author information
Authors and Affiliations
Corresponding author
Additional information
Software information: WINDOWS operating system, WORD word processing, SigmaPlot diagrams exported in tiff format.
Rights and permissions
About this article
Cite this article
Leyx, C., Van Miltenburg, J.C., Chopin, C. et al. Heat-capacity measurements and absolute entropy of ɛ-Mg2PO4OH. Phys Chem Minerals 32, 13–18 (2005). https://doi.org/10.1007/s00269-004-0432-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-004-0432-9