Abstract
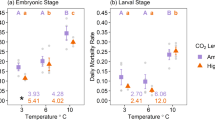
Salinity is a common stressor restricting the distribution of various decapod crustaceans. The interactive effects of such regional stressors with global climate change drivers are important to be considered when aiming to realistically predict the potential of a species’ dispersal and further spread into new habitats. Within species, their larval stages commonly determine a species tolerance and with this their potential to invade and successfully develop a sustaining population. This laboratory study investigated the combined effect of salinity (6 levels, 10–25) and temperature (19 and 23 °C) on larval survival, development to megalopa, and feeding (in Zoea I, III, and V) of the decapod Hemigrapsus takanoi. Larval development and survival to megalopa were generally favored by increasing salinity. While no larva developed to the megalopa stage at 23 °C and a salinity of 16, in 19 °C some larvae could successfully develop under a salinity as low as 16. All larval stages fed generally more with increasing salinity and temperature, but there was no interaction between the two factors. The results revealed that the H. takanoi population from Kiel Fjord (southwestern Baltic Sea) is capable of completing its larval development under the current Kiel Fjord environmental conditions. The geographical spread of this H. takanoi population into the wider Baltic Proper may, however, be restricted mainly due to the inability to establish and maintain a self-sustaining population under lower salinity conditions. Furthermore, the projected desalination of the Baltic Sea together with rising temperatures due to global warming and heat waves in summer may likely exert additional stress to this existing population, unless H. takanoi adapts at appropriate rates.



Similar content being viewed by others
Data availability
All data used in this study will be made publicly available on PANGAEA (https://www.pangaea.de) following acceptance as required by the GEOMR Helmholtz Centre for Ocean Research Kiel regulations.
References
Anger K (1991) Effects of temperature and salinity on the larval development of the Chinese mitten crab Eriocheir sinensis (Decapoda: Grapsidae). Mar Ecol Prog Ser 72:103–110
Anger K (1996) Salinity tolerance of the larvae and first juveniles of a semiterrestrial grapsid crab, Armuses miersii (Rathbun). J Exp Mar Biol Ecol 202:205–223
Anger K (2003) Salinity as a key parameter in the larval biology of decapod crustaceans. Inver Reprod Dev 43(1):29–45
Anger K (2006) Contributions of larval biology to crustacean research: a review. Inver Reprod Dev 49:175–205
Anger K, Charmantier G (2000) Ontogeny of osmoregulation and salinity tolerance in a mangrove crab, Sesarma curacaoense (Decapods: Grapsidae). J Exp Mar Biol Ecol 251:265–274
Anger K, Spivak E, Luppi T (1998) Effects of reduced salinities on development and bioenergetics of early larval shore crab, Carcinus maenas. J Exp Mar Biol Ecol 220:287–304
Anger K, Spivak E, Luppi T, Bas C, Ismael D (2008) Larval salinity tolerance of the South American salt-marsh crab, Neohelice (Chasmagnathus) granulata : physiological constraints to estuarine retention, export and reimmigration. Helgol Mar Res 62:93–102
Arnberg M, Calsoi P, Spicer JI, Tandberg AHS, Nilsen M, Wetsterlund S, Bechmann RK (2013) Elevated temperatures elicits greater effects than decreased pH on the development, feeding and metabolism of northern shrimp (Pandalus borealis) larvae. Mar Biol 160:2037–2048
Bas CC, Spivak ED (2000) Effect of salinity on embryos of two southwestern Atlantic estuarine grapsid crab species in vitro. J Crustac Biol 20(4):647–656
Bates D, Maechler M, Bolker B, Walker S (2017) Linear Mixed-Effects Models using ‘Eigen’ and S4. R package version 1.1–13.http://cran.r-project.org/package=lme4
Berggren M, Karlsson R (2017) Invasiva, asiatiska krabbor längs Västkusten. Fauna Flora 112:22–26
Casties I, Seebens H, Briski E (2016) Importance of geographic origin for invasion success: A case study of the North and Baltic Seas versus the Great Lakes – St. Lawrence River region. Ecol Evol 6:8318–8329
Charmantier G (1998) Ontogeny of osmoregulation in crustaceans: a review. Inver Reprod Dev 33(2–3):177–190
Charmantier G, Anger K (1999) Ontogeny of osmoregulation in the palaemonid shrimp Palaemonetes argentinus (Crustacea: Decapoda). Mar Ecol Prog Ser 181:125–129
Charmantier G, Giménez L, Charmantier-Daures M, Anger K (2002) Ontogeny of osmoregulation, physiological plasticity, and larval export strategy in grapsid crab Chasmagnathus granulata (Crustacea, Decaopda). Mar Ecol Prog Ser 229:185–194
Cieluch U, Anger K, Aujoulat F, Buchholz F, Charmantier-Daures M, Charmantier G (2004) Ontogeny of osmoregulatory structures and functions in the green crab Carcinus maenas (Crustacea, Decapoda). J Exp Biol 207(2):325–336
Cowen RK, Sponaugle S (2009) Larval dispersal and marine population connectivity. Ann Rev Mar Sci 1(1):443–466
Crainiceanu CM, Ruppert D (2004) Likelihood ratio tests in linear mixed models with one variance component. J R Stat Soc Series B Statist Methodol 66:165–185
Crisp DJ (1976) Settlement responses in marine organisms. In: Newell RC (ed) Adaptations to environment: essays on the physiology of marine animals. Butterworths, London, pp 83–124
Cuesta JA, González-Gordillo JI (2020) Varunidae H, Milne-Edwards 1853, and Ocypodidae Rafinesque, 1815. ICES Identification Leaflets for Plankton No. 190. 19pp. http://doi.org/https://doi.org/10.17895/ices.pub.5995
De Grande FR, Granado P, Carretero FH, Costa TM (2018) Organic matter affects fiddler crab distribution? Results from field and laboratorial trials. Estuar Coast Shelf Sci 212:138–145
Díaz H, Bevilacqua M (1986) Larval development of Aratus pisonii (Milne Edwards) (Brachyura, Grapsidae) from marine and estuarine environments reared under different salinity conditions. J Coast Res 2:43–49
Díaz H, Bevilacqua M (1987) Early developmental sequences of Aratus pisonii (H. Milne Edwards) (Brachyura, Grapsidae) under laboratory conditions. J Coast Res 3(1):63–70
Epifanio CE, Dittel AI, Park S, Schwalm S, Fouts A (1998) Early life history of Hemigrapsus sanguineus a non-indigenous crab in the Middle Atlantic Bight (USA). Mar Ecol pro Ser 170:231–238
Franz M, Barboza FR, Hinrichsen H-H, Lehmann A, Scotti M, Hiebenthal C, Molis M, Schütt R, Wahl M (2019) Long-term records of hard-bottom communities in the southwestern Baltic Sea reveal the decline of a foundation species. Estuar Coast Shelf Sci 219:242–251
Geburzi JC, Graumann G, Köhnk S, Brandis D (2015) First record of the Asian crab Hemigrapsus takanoi Asakura & Watanabe, 2005 (Decapoda, Brachyura, Varunidae) in the Baltic Sea. BioInvasions Rec 4:103–107
Giménez L (2010) Relationships between habitat conditions, larval traits, and juvenile performance in a marine invertebrate. Ecology 91:1401–1413
Giménez L, Anger K (2003) Larval performance in an estuarine crab, Chasmagnathus granulata, is a consequence of both larval and embryonic experience. Mar Ecol Prog Ser 249:251–264
Giménez L, Torres G (2002) Larval growth in the estuarine crab Chasmagnathus granulata: the importance of salinity experienced during embryonic development, and the initial larval biomass. Mar Biol 141(5):877–885
Giménez L, Anger K, Torres G (2004) Linking life history traits in successive phases of a complex life cycle: effects of larval biomass on early juvenile development in an estuarine crab, Chasmagnathus granulata. Oikos 104:570–580
Gimenez L, Anger K (2001) Relationships among salinity, egg size, embryonic development, and larval biomass in the estuarine crab Chasmagnathus granulata Dana, 1851. J Exp Mar Biol Ecol 260:241–257
Gollasch S (1999) The Asian decapod Hemigrapsus penicillatus (de Haan, 1835) (Grapsidae, Decapoda) introduced in European waters: status quo and future perspective. Helgol Meeres 52:359–366
Gonçalves F, Ribeiro R, Soares A (1995) Laboratory study of effects of temperature and salinity on survival and larval development of a population of Rhithropanopeus harrisii from the Mondego River estuary, Portugal. Mar Biol 121:639–645
González-Ortegón E, Giménez L (2014) Environmentally mediated phenotypic links and performance in larvae of a marine invertebrate. Mar Ecol pro Ser 502:185–195
Gräwe U, Friedland R, Burchard H (2013) The future of the western Baltic Sea: two possible scenarios. Ocean Dyn 63:901–921
Hernández JE, Palazón-Fernández JL, Hernández G, Bolaños J (2012) The effect of temperature and salinity on the larval developemnt of Stenorhynchus seticornis (Brachyura: Inachidae) reared in the laboratory. J Mar Biol Assoc UK 92(3):505–513
Holopanien R, Lehtiniemi M, Miere HEM, Albertsson J, Gorokhova E, Kotta J, Viitasalo M (2016) Impacts of changing climate on the non-indigenous invertebrates in the northern Baltic Sea by the end of the twenty-first century. Biol Invasions 18:3015–3032
Hughes AR, Stachowicz JJ (2004) Genetic diversity enhances the resistance of a seagrass ecosystem to disturbance. PNAS: 8998–9002
Jackson TD, Torres G, Giménez, (2014) Survival and development of larvae of two decapod crustaceans under limited access to prey across a thermal range. J Plankton Res 36(6):1476–1487
Janssen F, Schrum C, Backhaus JO (1999) A climatological data set of temperature and salinity for the Baltic Sea and the North Sea. De Hy Z 51:5–245
Johns DM (1982) Physiological studies on Cancer irroratus larvae. III. Effects of temperature and salinity on the partitioning of energy resources during development. Mar Ecol Prog Ser 8:75–85
Koram SS (2019) Key concept of quadratic functions and Inequalities-First Edition: Springer Series. Geomech Geoeng 13(1):9–22
Landeira JS, Cuesta JA, Tanaka Y (2019) Larval development of the brush-clawed shore crab Hemigrapsus takanoi Asakura & Watanabe, 2005 (Decapoda, Brachyura, Varunidae). J Mar Biol Assoc UK 99(5):1153–1164
Lárez MB, Palazón-Fernández JL, Bolaños CJ (2000) The effect of salinity and temperature on the larval development of Mithrax caribbaeus Rathbun, 1920 (Brachyura: Majidae) reared in the laboratory. J Palnkton Res 22:1855–1869
Meier HEM, Hordoir R, Andersson HC, Dieterich C, Eilola K, Gustafsson BG, Höglund A, Schimanke S (2012) Modeling the combined impact of changing climate and changing nutrient loads on the Baltic Sea environment in an ensemble of transient simulations for 1961–2099. Clim Dyn 39:2421–2441
Mingkid WM, Awika S, Watanabe S (2006a) Morphological characteristics, pigmentation, and distribution of the sibling penicillate crabs, Hemigrapsus penicillatus (de Haan 1835) and H. takanoi Asakura & Watanabe, 2005 (Decapoda, Brachyura, Grapsidae) in Tokyo bay. Crustaceana 79:1107–1121
Mingkid WM, Masashi Yokota M, Watanabe S (2006b) Salinity tolerance of larvae in the penicillate crab Hemigrapsus takanoi (Decapoda: Brachyura: Grapsidae). La Mer 44:17–21
Moksnes P-O (2004) Interference competition for space in nursery habitats: density-dependent effects on growth and dispersal in juvenile shore crabs Carcinus maenas. Mar Ecol Prog Ser 281:181–191
Montú IM, Anger K, de Bakker C (1990) Variability in the larval development of Metasesarma rubripes (Decapoda, Grapsidae) reared in the laboratory. Neritica. Pontal Do Sul PR (brazil) 5:113–128
Morgan SG (1995) Life and death in the plankton: larval mortality and adaptation. In: McEdward L (ed) Ecology of marine invertebrate larvae. CRC Press, Boca Raton, FL, pp 279–321
Müller JD, Schneider B, Rehder G (2016) Long-term alkalinity trends in the Baltic Sea and their implictaions for CO2-induced acidification. Limnol Oceanogr 61:1984–2002
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142
Nour OM, Pansch C, Lenz M, Wahl M, Clemmesen C, Stumpp M (2021) Impaired larval development at low salinities could limit the spread of the non-native crab Hemigrapsus takanoi in the Baltic Sea. Aquat Biol 30:85–99
Paavola M, Olenie S, Leppäkoski E (2005) Are invasive species most successful in habitats of low native species richness across European brackish water seas? Estuar Coast Shelf Sci 64(4):738–750
Paiva F, Barco A, Chen Y, Mirzajani A, Chan FT, Lauringson V, Baltazar-Soares M et al (2018) Is salinity an obstacle for biological invasions? Glob Change Biol 24(6):2708–2720
Palumbi SR (2003) Population genetics, demographic connectivity, and the design of marine reserves. Ecol Appl 13(1):146–158
Pansch C, Scotti M, Barboza FR, Al-Janabi B, Brakel J, Briski E et al (2018) Heat waves and their significance for a temperate benthic community: a near-natural experimental approach. Glob Change Biol 24:4357–4367. https://doi.org/10.1111/gcb.14282
Parshin-Chudin AV, Borisov RR, Kovacheva NP, Lebedev RO, Nazartseva MY (2014) Effect of salinity on the survival of the red king crab; Paralithodes camtschaticus (Tilesius, 1815), at early stages of ontogeny. Russ J Ecol 45(2):150–152
Paul AJ, Nunes P (1983) Temperature modification of respiratory metabolism and caloric intake of Pandalus borealis (Krøyer) first zoeae. J Exp Mar Biol Ecol 66:163–168
Qiu J, Qian P (1999) Tolerance of the barnacle Balanus amphitrite to salinity and temperature stress: effects of previous experience. Mar Ecol Prog Ser 188:123–132
R Core Team (2016) R: a language and environment for statistical computing.https://www.Rproject.org
Reusch TBH, Dierking J, Andersson HC, Bonsdorff E, Carstensen J, Casini M et al (2018) The Baltic Sea as a time machine for the future coastal ocean. Sci Adv. https://doi.org/10.1126/sciadv.aar8195
Roger F, Godhe A, Gamfeldt L (2012) Genetic diversity and ecosystem functioning in the face of multiple stressors. PLoS ONE 7(9):e45007
Rosenberg R, Costlow J (1979) Delayed response to irreversible non-genetic adaptation to salinity in early development of the brachyuran crab Rhithropanopeus harrisii, and some notes on adaptation to temperature. Ophelia 18:97–112
Sastry AN (1983) Ecological aspects of reproduction. In: Vernberg FJ, Vernberg WB (eds) The Biology of Crustacea, vol 8. Environmental Adaptations. Academic Press, New York, pp 179–270
Schiffer M, Harms L, Lucassen M, Mark FC, Pörtner H-O, Storch D (2014) Temperature tolerance to different larval stages of the spider crab Hyas araneus exposed to elevated seawater PCO2. Front Zool 11:87
Selkoe KA, D’Aloia CC, Crandall ED, Iacchei M, Liggins L, Puritz JB, Von Der Heyden S, Toonen RJ (2016) A decade of seascape genetics: contributions to basic and applied marine connectivity. Mar Ecol Prog Ser 554:1–19
Simith DJB, de Souza AS, Maciel CR, Fernando M, Abrunhosa FA, Diele K (2012) Influence of salinity on the larval development of the fiddler crab Uca vocator (Ocypodidae) as an indicator of ontogenetic migration towards off shore waters. Helgol Mar Res 66:77–85
Skinner DM (1985) Molting and regeneration. In: Bliss D, Mantel LH (eds) The biology of Crustacea, vol 9. Academic Press, New York, pp 43–146
Soors J, Faasse M, Stevens M, Verbessem I, de Regge N, Van den Bergh E (2010) New crustacean invaders in the Schelde estuary (Belgium). Belg J Zool 140:3–10
Thomsen J, Ramesh K, Sanders T, Belich M, Melzner F (2018) Calcification in a marginal sea- influence of seawater [Ca2+] and carbonate chemistry on bivalve shell formation. Biogeosciences 15:1469–1482
Torres G, Giménez L, Anger A (2007) Effects of osmotic stress on crustacean larval growth and protein and lipid levels are related to life-histories: The genus Aramses as a model. Comp Biochem Phys B 148:209–224
Tsiamis K, Palialexis A, Stefanova K, Gladan ZN, Skejić S et al (2019) Non-indigenous species refined national baseline inventories: a synthesis in the context of the European Union’s Marine Strategy Framework Directive. Mar Pollut Bull 145:429–435
van den Brink A, Godschalk M, Smaal A, Lindeboom H, McLay C (2013) Some like it hot: the effect of temperature on brood development in the invasive crab Hemigrapsus takanoi (Decapoda: Brachyura: Varunidae). J Mar Biol Associ UK 93:189–196
Vuorinen I, Hännine J, Rajasilta M, Laine P, Eklund J, Montesino-Pouzols CF, Junker K, Meier HEM, Dippner JW (2015) Scenario simulations of future salinity and ecological consequence in the Baltic Sea and adjacent North Sea areas-implications of environmental monitoring. Ecol Indic 50:196–205
Wahl M, Schneider Covachã S, Saderne V, Hiebenthal C, Müller JD, Pansch C et al (2017) Macroalgae may mitigate ocean acidification effects on mussel calcification by increasing pH and its fluctuations. Limnol Oceanogr 63:3–21
Walther K, Anger K, Pörtner HO (2010) Effects of ocean acidification and warming on the larval development of the spider crab Hyas araneus from different latitudes (54° vs. 79°N). Mar Ecol Prog Ser 417:159–170
Weersing K, Toonen RJ (2009) Population genetics, larval dispersal, and connectivity in marine systems. Mar Ecol Prog Ser 393:1–12
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer, New York
Wolf F, Seebass K, Pansch C (2022) The role of recovery phases in mitigating the negative impacts of marine heatwaves on the sea star Asterias rubens. Front Mar Sci 8:79024. https://doi.org/10.3389/fmars.2021.790241
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1:3–14
Acknowledgements
We thank Dr. Gabriela Torres for giving advice for larvae rearing techniques, Björn Buchholz for technical assistance, Prof. Dr. Martin Wahl for his helpful advice, and Dr. Mark Lenz for statistical advice.
Funding
OMN acknowledges the financial support of the German Academic Exchange Service (DAAD) through the project German Egyptian Research Long-term Scholarship Programme (GERLS) 2015/16 (57147166). MS was funded through the German research foundation—Deutsche Forschungsgesellschaft (DFG) STU 715/2-1 (441084746).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Ethical statement
This study was carried out and funded by GEOMAR Helmholtz Centre for Ocean Research Kiel, Germany, with no conflict of interest. All applicable international, national, and/or institutional guidelines for sampling, care, and experimental use of organisms for the study have been followed, applying methods and protocols approved by the regulatory committee on the ethic of animal experiments, of Schleswig–Holstein Germany under the permit number: 1101, and in strict accordance to the relevant regulations and guidelines. All possible actions were taken to reduce animal suffering and stress during handling and sampling.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nour, O.M., Pansch, C. & Stumpp, M. Freshening and warming may restrict dispersal of Hemigrapsus takanoi into the Baltic Proper due to interactive effects on larval survival and feeding. Mar Biol 169, 125 (2022). https://doi.org/10.1007/s00227-022-04112-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-022-04112-0