Abstract
Planktonic primary consumers have been shown to strongly influence phytoplankton communities via top-down effects such as grazing and nutrient recycling. However, it remains unclear how changes in consumer richness may alter the stoichiometric constrains between producer and consumer assemblages. Here we test whether the stoichiometry of producer–consumer interactions is affected by the species richness of the consumer community (multispecies consumer assemblage vs single consumer species). Therefore, we fed a phytoplankton assemblage consisting of two flagellates and two diatom species reared under a 2 × 2 factorial combination of light and nitrogen supply to three planktonic consumer species in mono- and polycultures. As expected, phytoplankton biomass and C:nutrient ratios significantly increased with light intensity while nitrogen limitation resulted in reduced phytoplankton biomass and increasing phytoplankton C:N but lower N:P. Differences in phytoplankton stoichiometry were partly transferred to the consumer level, i.e., consumer C:N significantly increased with phytoplankton C:N. Consumer diversity significantly increased consumer biomass, resource use efficiency and nutrient uptake. In turn, consumer N:P ratios significantly decreased in consumer assemblages under high resource supply due to unequal changes in nutrient uptake. Consumer diversity further altered phytoplankton biomass, stoichiometry and species composition via increased consumption. Whether the effects of consumer diversity on phytoplankton and consumer performance were positive or negative strongly depended on the resource supply. In conclusion, the stoichiometric constraints of trophic interactions in multispecies assemblages cannot be predicted from monoculture traits alone, but consumer diversity effects are constrained by the resources supplied.




Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Change history
01 February 2020
Unfortunately, in the print version of this article the below appendix
References
Boersma M, Aberle N, Hantzsche FM, Schoo KL, Wiltshire KH, Malzahn AM (2008) Nutritional limitation travels up the food chain. Int Rev Hydrobiol 93:479–488. https://doi.org/10.1002/iroh.200811066
Bruno JF, Cardinale BJ (2008) Cascading effects of predator richness. Front Ecol Environ 6:539–546
Bruno JF, Boyer KE, Duffy JE, Lee SC (2008) Relative and interactive effects of plant and grazer richness in a benthic marine community. Ecology 89:2518–2528
Burson A, Stomp M, Akil L, Brussaard CPD, Huisman J (2016) Unbalanced reduction of nutrient loads has created an offshore gradient from phosphorus to nitrogen limitation in the North Sea. Limnol Oceanogr 61:869–888
Cardinale BJ, Harvey CT, Gross K, Ives AR (2003) Biodiversity and biocontrol: emergent impacts of a multi-enemy assemblage on pest suppression and crop yield in an agroecosystem. Ecol Lett 6:857–865
Cardinale BJ, Hillebrand H, Harpole WS, Gross K, Ptacnik R (2009) Separating the influence of resource ‘availability’ from resource ‘imbalance’ on productivity–diversity relationships. Ecol Lett 12:475–487
Cardinale BJ, Duffy JE, Gonzalez A, Hooper DU, Perrings C, Venail P, Narwani A, Mace GM, Tilman D, Wardle DA, Kinzig AP, Daily GC, Loreau M, Grace JB, Larigauderie A, Srivastava DS, Naeem S (2012) Biodiversity loss and its impact on humanity. Nature. 486:59–67. https://doi.org/10.1038/nature11148
Carnicer J, Sardans J, Stefanescu Cm Ubach A, Bartrins M, Asensio D, Peñuelas J (2015) Global biodiversity, stoichiometry and ecosystem responses to human-induced C–N–P imbalances. J Plant Physiol 172:82–91
Chen M, Fan M, Kuang Y (2017) Global dynamics in a stoichiometric food chain model with two limiting nutrients. Math Biosci 289:9–19
Doi H, Cherif M, Iwabuchi T, Katano I, Stegen JC, Striebel M (2010) Integrating elements and energy through the metabolic dependencies of gross growth efficiency and the threshold elemental ratio. Oikos 119:752–765
Doney SC (2010) The growing human footprint on coastal and open-ocean biogeochemistry. Science 328:1512–1516
Droop MR (1973) Some thoughts on nutrient limitation in algae. J Phycol 9:264–272
Duffy JE (2002) Biodiversity and ecosystem function: the consumer connection. Oikos 99:201–219
Duffy JE (2003) Biodiversity loss, trophic skew and ecosystem functioning. Ecol Lett 6:680–687
Duffy JE, Cardinale BJ, Kristin EF, McIntire PB, Thebault E, Loreau M (2007) The functional role of biodiversity in ecosystems: incorporating trophic complexity. Ecol Lett 10:522–538
Elrifi IR, Turpin DH (1985) Steady state luxury consumption and the concept of optimum nutrient ratios: a study with phosphate and nitrate limited Selenastrum minutum (Chlorophyta). J Phycol 21:592–602
Elser JJ, Bennet E (2011) Phosphorus cycle: a broken biogeochemical cycle. Nature 478:29
Elser JJ, Urabe J (1999) The stoichiometry of consumer-driven nutrient recycling: theory, observations, and consequences. Ecology 80:735–751
Elser JJ, Frost P, Kyle M, Urabe J, Andersen T (2002) Effects of light and nutrients on plankton stoichiometry and biomass in a P-limited lake. Hydrobiology 481:101–112
Elser JJ, Andersen T, Baron JS, Bergström AK, Jansson M, Kyle M, Nydick KR, Steger L, Hessen DO (2009) Shifts in lake N:P stoichiometry and nutrient limitation driven by atmospheric nitrogen deposition. Science 326:835–837
Elser JJ, Loladze I, Peace AL, Kuang Y (2012) Lotka re-loaded: modelling trophic interactions under stoichiometric constraints. Ecol Model 245:3–11
Elser JJ, Kyle M, Learned J, McCrackin ML, Peace AL, Steger L (2016) Life on the stoichiometric knife-edge: effects of high and low food C: p ratio on growth, feeding, and respiration in three Daphnia species. Inland Waters 6:136–146
Filip J, Bauer B, Hillebrand H, Beniermann A, Gaedke U, Moorthi SD (2014) Multitrophic diversity effects depend on consumer specialization and species-specific growth and grazing rates. Oikos 123:912–922. https://doi.org/10.1111/oik.01219
Fox JW (2004) Effects of phytoplankton and herbivore diversity on the partitioning of biomass within and among trophic levels. Ecology 85:549–559
Frost PC, Benstead JP, Cross WF, Hillebrand H, Larson JH, Xenopoulos MA, Yoshida T (2006) Threshold elemental ratios of carbon and phosphorus in aquatic consumers. Ecol Lett 9:774–779. https://doi.org/10.1111/j.1461-0248.2006.00919.x
Gamfeldt L, Hillebrand H (2011) Effects of total resources, resource ratios, and species richness on phytoplankton productivity and evenness at both metacommunity and local scales. PLoS One 6:e21972
Gamfeldt L, Hillebrand H, Jonsson PR (2005) Species richness changes across two trophic levels simultaneously affect prey and consumer biomass. Ecol Lett 8:696–703
Grasshoff K, Kremling K, Ehrhardt M (1999) Methods of seawater analysis, 3rd completely revised and extended edition. Wiley-VCH, Weinheim
Grizzetti B, Bouraoui F, Aloe A (2012) Changes of nitrogen and phosphorus loads to European seas. Glob Change Biol 18:769–782
Gross K, Cardinale BJ (2007) Does species richness drive community production or vice versa? Reconciling historical and contemporary paradigms in competitive communities. Am Nat 170:207–220
Guillard RRL, Ryther JH (1962) Studies of marine planktonic diatoms: i. Cyclotella nana hustedt, and Detonula confervacea (Cleve) gran. Can J Microbiol 8:229–239
Hall SR (2009) Stoichiometrically explicit food webs: feedbacks between resource supply, elemental constraints, and species diversity. Annu Rev Ecol Evol Syst 40:503–528
Hall SR, Leibold MA, Lytle DA, Smith VH (2004) Stoichiometry and planktonic grazer composition over gradients of light, nutrients and predation risk. Ecology 85:2291–2301
Hector A, Bazeley-White E, Loreau M, Otway S, Schmid B (2002) Overyielding in grassland communities: testing the sampling effect hypothesis with replicated biodiversity experiments. Ecol Lett 5:502–511. https://doi.org/10.1046/j.1461-0248.2002.00337.x
Hessen DO, Elser JJ, Sterner RW, Urabe J (2013) Ecological stoichiometry: an elementary approach using basic principles. Limnol Oceanogr 58:2219–2236
Hillebrand H, Cardinale BJ (2004) Consumer effects decline with prey diversity. Ecol Lett 7:192–201
Hillebrand H, Lehmpfuhl V (2011) Resource stoichiometry and consumers control the biodiversity–productivity relationship in pelagic metacommunities. Am Nat 178:171–181
Hillebrand H, Dürselen CD, Kirschtel D, Pollingher U, Zohary T (1999) Biovolume calculation for pelagic and benthic microalgae. J Phycol 35:403–424
Hillebrand H, Frost P, Liess A (2008) Ecological stoichiometry of indirect grazer effects on periphyton nutrient content. Oecologia 155:619–630
Hillebrand H, Gamfeldt L, Jonsson PR, Matthiessen B (2009) Consumer diversity indirectly changes prey nutrient content. Mar Ecol Prog Ser 380:33–41
Hillebrand H, Cowles JM, Lewandowska A, Van de Waal DB, Plum C (2014) Think ratio! A stoichiometric view on biodiversity–ecosystem functioning research. Basic Appl Ecol 15:465–474
Hood JM, Sterner RW (2014) Carbon and phosphorus linkages in Daphnia growth are determined by growth rate, not species or diet. Funct Ecol 28:1156–1165. https://doi.org/10.1111/1365-2435.12243
Hooper DU, Chapin FS, Ewel JJ, Hector A, Inchausti P, Lavorel S, Lawton JH, Lodge DM, Loreau M, Naeem S, Schmid B, Setälä H, Symstad AJ, Vandermeer J, Wardle DA (2005) Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol Monogr 75:3–35
Ives AR, Cardinale BJ, Snyder WE (2005) A synthesis of subdisciplines: predator–prey interactions, and biodiversity and ecosystem functioning. Ecol Lett 8:102–116
Jaschinski S, Aberle N, Gohse-Reimann S, Brendelberger H, Wiltshire KH, Sommer U (2009) Grazer diversity effects in an eelgrass–epiphyte–microphytobenthos system. Oecologia 159:607–615
Jolliffe PA (2000) The replacement series. J Ecol 88:371–385. https://doi.org/10.1046/j.1365-2745.2000.00470.x
Knoll LB, McIntyre PB, Vanni MJ, Flecker AS (2009) Feedbacks of consumer nutrient recycling on producer biomass and stoichiometry: separating direct and indirect effects. Oikos 118:1732–1742. https://doi.org/10.1111/j.1600-0706.2009.17367.x
Kraberg A, Bauman M, Dürselen CD (2010) Coastal Phytoplankton. Photo guide for Northern European Seas. Friedrich Pfeil, München
Liess A, Haglund AL (2007) Periphyton responds differentially to nutrients recycled in dissolved or faecal pellet form by the snail grazer Theodoxus fluviatilis. Freshw Biol 52:1997–2008
Loladze I, Kuang Y, Elser JJ, Fagan WF (2004) Competition and stoichiometry: coexistence of two predators on one prey. Theor Popul Biol 65:1–15
Loreau M, Hector A (2001) Partitioning selection and complementarity in biodiversity experiments. Nature 412:72–76
Mace GM, Norris K, Fitter AH (2012) Biodiversity and ecosystem services: a multilayered relationship. Trends Ecol Evol 27:19–26. https://doi.org/10.1016/j.tree.2011.08.006
Malzahn AM, Boersma M (2012) Effects of poor food quality on copepod growth are dose dependent and non-reversible. Oikos 121:1408–1416
Malzahn AM, Aberle N, Clemmensen C, Boersma M (2007) Nutrient limitation of primary producers affects planktivorous fish condition. Limnol Oceanogr 52:2062–2071
Malzahn AM, Hantzsche F, Schoo KL, Boersma M, Aberle N (2010) Differential effects of nutrient-limited primary production on primary, secondary or tertiary consumers. Oecologia 162:35–48
Meunier CL, Haafke J, Oppermann B, Boersma M, Malzahn AM (2012) Dynamic stoichiometric response to food quality fluctuations in the heterotrophic dinoflagellate Oxyrrhis marina. Mar Biol 159:2241–2248. https://doi.org/10.1007/s00227-012-2009-3
Moorthi SI, Hillebrand H, Wahl M, Berninger UG (2008) Consumer diversity enhances secondary production by complementarily effects in experimental ciliate assemblages. Estua Coasts 31:152–162
O´Connor NE, Donohue I (2013) Environmental context determines multi-trophic effects of consumer species loss. Glob Change Biol 19:431–440
O´Connor MI, Gonzalez A, Byrnes JEK, Cardinale BJ, Duffy JE, Gamfeldt L, Griffin JN, Hooper D, Hungate BA, Paquette A, Thompson PL, Dee LE, Dolan KL (2017) A general biodiversity-function relationship is mediated by trophic level. Oikos 126:18–31
Peace AL, Zhao Y, Loladze I, Elser JJ, Kuang Y (2013) A stoichiometric producer-grazer model incorporating the effects of excess food-nutrient content on consumer dynamics. Math Biosci 244:107–115
Peñuelas J et al (2012) The human-induced imbalance between C, N and P in earth’s life system. Glob Change Biol 18:3–6
Persson J et al (2010) To be or not to be what you eat: regulation of stoichiometric homeostasis among autotrophs and heterotrophs. Oikos 119:741–751
Plum C, Hüsener M, Hillebrand H (2015) Multiple vs single phytoplankton species alter stoichiometry of trophic interaction with zooplankton. Ecology 96:3075–3089
Ptacnik R et al (2008) Diversity predicts stability and resource use efficiency in natural phytoplankton communities. Proc Nat Acad Sci 105:5134–5138
Sardans J, Rivas-Ubach A, Peñuelas J (2012) The C:N:P stoichiometry of organisms and ecosystems in a changing world: a review and perspectives. Perspect Plant Ecol Evol Syst 14:33–47
Schoo KL, Aberle N, Malzahn AM, Boersma M (2010) Does the nutrient stoichiometry of primary producers affect the secondary consumer Pleurobrachia pileus? Aquat Ecol 44:233–242. https://doi.org/10.1007/s10452-009-9265-4
Sih A, Englund G, Wooster D (1998) Emergent impacts of multiple predators on prey. Trends Ecol Evol 13:350–355. https://doi.org/10.1016/S0169-5347(98)01437-2
Snaydon R (1991) Replacement or additive designs for competition studies? J Appl Ecol 28:930–946
Sterner RW (1990) The ratio of nitrogen to phosphorus resupplied by herbivores: zooplankton and the algal competitive arena. Am Nat 136:209–229
Sterner R, Elser J (2002) Ecological stoichiometry. Princeton University Press, Princeton, New Jersey, USA, The biology of elements from molecules to the biosphere
Sterner RW et al (1997) The light:nutrient ratio in lakes: The balance of energy and materials affects ecosystem structure and process. Am Nat 150:663–684
Striebel M, Spörl G, Stibor H (2008) Light-induced changes of plankton growth and stoichiometry: experiments with natural phytoplankton communities. Limnol Oceanogr 53:513–522
Van de Waal DB, Elser JJ, Martiny AC, Sterner RW, Cotner JB (2018) Progress in ecological stoichiometry. Front Microbiol. https://doi.org/10.3389/fmicb.2018.01957
Van Nieuwerburgh L et al (2004) Growth and C:N: P ratios in copepods grazing on N- or Si-limited phytoplankton blooms. Hydrobiol 514:57–72
Vitousek PM et al (2010) Terrestrial phosphorus limitation: mechanisms, implications, and nitrogen–phosphorus interactions. Ecol Appl 20:5–15
Welti N, Striebel M, Ulseth AJ, Cross WF, DeVilbiss S, Glibert PM, Guo L, Hirst AG, Hood J, Kominoski JS, MacNeill KL, Mehring AS, Welter JR, Hillebrand H (2017) Bridging food webs, ecosystem metabolism, and biogeochemistry using ecological stoichiometry theory. Front Microbiol 8:1298. https://doi.org/10.3389/fmicb.2017.01298
Acknowledgements
We thank Heike Rickels for technical support, as well as Mareen Möller and Samuel Nietzer for their support during the experiment. We additionally thank Maren Striebel, Stefanie Moorthi and Thomas Anderson for their competent and fruitful feedback on the manuscript. We also thank two anonymous reviewers for their helpful comments on the manuscript.
Funding
This study was funded by the German Research Council (Deutsche Forschungsgemeinschaft DFG Hi 848 7-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed by the authors.
Additional information
Responsible Editor: R. Bi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewed by C. Laspoumaderes and undisclosed experts.
Appendix
Appendix
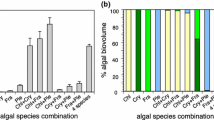
See Table 4, and Figs. 5, 6 and 7.
Skeletonema costatum (Ske) was proportionally the most abundant species in the algal assemblages (except for HL +N) with up to 70% of the total biovolume. Both, Rhodomonas salina (Rho) and Tetraselmis sp. (Tet) remained below 20% of total biovolume. The proportions were more equally distributed at HL and +N which was mainly based on the dominance of Tetraselmis sp. (up to 50% of total biovolume) and higher proportions of the subdominant species Rhodomonas salina (up to 25% of total biovolume). The biovolume of Chaetoceros danicus (Chae) contributed less than 1% to the total algal biovolume under all resource conditions
Rights and permissions
About this article
Cite this article
Plum, C., Hillebrand, H. Multiple zooplankton species alter the stoichiometric interactions between producer and consumer levels. Mar Biol 166, 163 (2019). https://doi.org/10.1007/s00227-019-3609-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-019-3609-y