Abstract
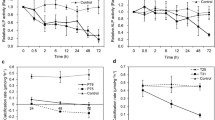
Ocean acidification (OA) is beginning to have noticeable negative impact on calcification rate, shell structure and physiological energy budgeting of several marine organisms; these alter the growth of many economically important shellfish including oysters. Early life stages of oysters may be particularly vulnerable to OA-driven low pH conditions because their shell is made up of the highly soluble form of calcium carbonate (CaCO3) mineral, aragonite. Our long-term CO2 perturbation experiment showed that larval shell growth rate of the oyster species Crassostrea hongkongensis was significantly reduced at pH < 7.9 compared to the control (8.2). To gain new insights into the underlying mechanisms of low-pH-induced delays in larval growth, we have examined the effect of pH on the protein expression pattern, including protein phosphorylation status at the pediveliger larval stage. Using two-dimensional electrophoresis and mass spectrometry, we demonstrated that the larval proteome was significantly altered by the two low pH treatments (7.9 and 7.6) compared to the control pH (8.2). Generally, the number of expressed proteins and their phosphorylation level decreased with low pH. Proteins involved in larval energy metabolism and calcification appeared to be down-regulated in response to low pH, whereas cell motility and production of cytoskeletal proteins were increased. This study on larval growth coupled with proteome change is the first step toward the search for novel Protein Expression Signatures indicative of low pH, which may help in understanding the mechanisms involved in low pH tolerance.










Similar content being viewed by others
References
Amaral V, Cabral HN, Bishop MJ (2011) Resistance among wild invertebrate populations to recurrent estuarine acidification. Estuar Coast Shelf Sci 93:460–467
Barton A, Hales B, Waldbusser GG, Langdon C, Feely RA (2012) The Pacific oyster, Crassostrea gigas, shows negative correlation to naturally elevated carbon dioxide levels: implications for near-term ocean acidification effects. Limnol Oceanogr 57:698–710
Beranova-Giorgianni S (2003) Proteome analysis by two-dimensional gel electrophoresis and mass spectrometry: strengths and limitations. TrAC-Trends Anal Chem 22:273–281
Bibby R, Widdicombe S, Parry H, Spicer J, Pipe R (2008) Effects of ocean acidification on the immune response of the blue mussel Mytilys edulis. Aquat Biol 2:67–74
Breese WP, Malouf RE (1975) Hatchery manual for the Pacific oyster ORESU-H-75-002. Oregon State University Sea Grant College Program, Corvallis
Byrne M (2011) Impact of ocean warming and ocean acidification on marine invertebrate life history stages: vulnerabilities and potential for persistence in a changing ocean. Oceanogr Mar Biol Annu Rev 49:1–42
Candiano G, Bruschi M, Musante L, Santucci L, Ghiggeri GM, Carnemolla B, Orecchia P, Zardi L, Righetti PG (2004) Blue silver: a very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis 25:1327–1333
Carpentier SC, Panis B, Vertommen A, Swennen R, Sergeant K, Renaut J, Laukens K, Witters E, Samyn B, Devreese B (2008) Proteome analysis of non-model plants: a challenging but powerful approach. Mass Spectrom Rev 27:354–377
Casado-Vela J, Cebrián A, Gómez del Pulgar MT, Sánchez-López E, Vilaseca M, Menchén L, Diema C, Sellés-Marchart S, Martínez-Esteso MJ, Yubero N, Bru-Martínez R, Lacal JC (2011) Lights and shadows of proteomic technologies for the study of protein species including isoforms, splicing variants and protein post-translational modifications. Proteomics 11:590–603
Chandramouli K, Mok F, Wang H, Qian P-Y (2011) Phosphoproteome analysis during larval development and metamorphosis in the spionid polychaete Pseudopolydora vexillosa. BMC Dev Biol 11:31
Chich JF, David O, Villers F, Schaeffer B, Lutomski D, Huet S (2007) Statistics for proteomics: experimental design and 2-DE differential analysis. J Chromatogr B 849:261–272
Comparot S, Lingiah G, Martin T (2003) Function and specificity of 14–3-3 proteins in the regulation of carbohydrate and nitrogen metabolism. J Exp Bot 54:595–604
Deboer ML, Krupp DA, Weis VM (2007) Proteomic and transcriptional analyses of coral larvae newly engaged in symbiosis with dinoflagellates. Comp Biochem Physiol Part D 2:63–73
Dickson AG (1981) An exact definition of total alkalinity and a procedure for the estimation of alkalinity and total inorganic carbon from titration data. Deep Sea Res Part A Oceanogr Res Pap 28:609–623
Dineshram R, Wong KKW, Xiao S, Yu Z, Qian PY, Thiyagarajan V (2012) Analysis of Pacific oyster larval proteome and its response to high-CO2. Mar Pollut Bull 64:2160–2167. doi:10.1016/j.marpolbul.2012.07.043
Diz AP, Dudley E, Skibinski DOF (2012a) Identification and characterization of highly expressed proteins in sperm cells of the marine mussel Mytilus edulis. Proteomics 12:1949–1956
Diz AP, Martinez-Fernandez M, Rolan-Alvarez E (2012b) Proteomics in evolutionary ecology: linking the genotype with the phenotype. Mol Ecol 21:1060–1080
Dupont S, Thorndyke MC (2009) Impact of CO2-driven ocean acidification on invertebrates early life-history: what we know, what we need to know and what we can do. Biogeosci Disc 6:3109–3131
Feely RA, Orr J, Fabry VJ, Kleypas JA, Sabine CL, Langdon C (2009) Present and future changes in seawater chemistry due to ocean acidification. Geophys Monogr 183:175–188
Fu H, Subramanian RR, Masters SC (2000) 14-3-3 Proteins: structure, function, and regulation. Annu Rev Pharmacol Toxicol 40:617–647
Gazeau F, Quiblier C, Jansen JM, Gattuso JP, Middelburg JJ, Heip CHR (2007) Impact of elevated CO2 on shellfish calcification. Geophys Res Lett 34:L07603
Gazeau F, Gattuso JP, Dawber C, Pronker AE, Peene F, Peene J, Heip CHR, Middelburg JJ (2010) Effect of ocean acidification on the early life stages of the blue mussel (Mytilus edulis). Biogeosci Disc 7:2927–2947
Gazeau F, Gattuso JP, Greaves M, Elderfield H, Peene J, Heip CHR, Middelburg JJ (2011) Effect of carbonate chemistry alteration on the early embryonic development of the pacific oyster Crassostrea gigas. PLoS ONE 6:e23010
Gibson R, Atkinson R, Gordon J, Smith I, Hughes D (2011) Impact of ocean warming and ocean acidification on marine invertebrate life history stages: vulnerabilities and potential for persistence in a changing ocean. Oceanogr Mar Biol Annu Rev 49:1–42
Görg A, Weiss W, Dunn MJ (2004) Current two-dimensional electrophoresis technology for proteomics. Proteomics 4:3665–3685
Guinotte JM, Fabry VJ (2008) Ocean acidification and its potential effects on marine ecosystems. Ann N Y Acad Sci 1134:320–342
Havenhand JN, Schlegel P (2009) Near-future levels of ocean acidification do not affect sperm motility and fertilization kinetics in the oyster Crassostrea gigas. Biogeosciences 6:3009–3015
Hidalgo C (2005) Cross talk between Ca2+ and redox signalling cascades in muscle and neurons through the combined activation of ryanodine receptors/Ca2+ release channels. Philos Trans R Soc Lond B Biol Sci 360:2237–2246
Hofmann GE, O’Donnell MJ, Todgham AE (2008) Using functional genomics to explore the effects of ocean acidification on calcifying marine organisms. Mar Ecol Prog Ser 373:219–225
Huan P, Wang H, Dong B, Liu B (2012) Identification of differentially expressed proteins involved in the early larval development of the Pacific oyster Crassostrea gigas. J proteomics 75:3855–3865
Johnson S, Browman H, Hoffmann G, Place S, Dupont S, Wilson K, Obst M, Sköld H, Nakano H, Thorndyke M (2007) Introducing genomics, proteomics and metabolomics in marine ecology. Mar Ecol Prog Ser 332:247–248
Kaniewska P, Campbell PR, Kline DI, Rodriguez-Lanetty M, Miller DJ, Dove S, Hoegh-Guldberg O (2012) Major cellular and physiological impacts of ocean acidification on a reef building coral. PLoS ONE 7:e34659
Kültz D, Chakravarty D, Adilakshmi T (2001) A novel 14-3-3 gene is osmoregulated in gill epithelium of the euryhaline teleost Fundulus heteroclitus. J Exp Biol 204:2975–2985
Kurihara H (2008) Effects of CO2-driven ocean acidification on the early developmental stages of invertebrates. Mar Ecol Prog Ser 373:275–284
Kurihara H, Kato S, Ishimatsu A (2007) Effects of increased seawater pCO2 on early development of the oyster Crassostrea gigas. Aquat Biol 1:91–98
Lam K, Morton B (2004) The oysters of Hong Kong (Bivalvia: Ostreidae and Gryphaeidae). Raffles Bull Zool 52:11–28
Li S, Xie L, Meng Q, Zhang R (2006) Significance of the extra C-terminal tail of CaLP, a novel calmodulin-like protein involved in oyster calcium metabolism. Comp Biochem Physiol Part B 144:463–471
López JL (2007) Two-dimensional electrophoresis in proteome expression analysis. J Chromatogr B 849:190–202
López JL, Abalde SL, Fuentes J (2005) Proteomic approach to probe for larval proteins of the mussel Mytilus galloprovincialis. Mar Biotechnol 7:396–404
Marshall DJ, Morgan SG (2011) Ecological and evolutionary consequences of linked life-history stages in the sea. Curr Biol 21:R718–R725
Martin S, Richier S, Pedrotti ML, Dupont S, Castejon C, Gerakis Y, Kerros M-E, Oberhänsli F, Teyssié JL, Jeffree R, Gattuso JP (2011) Early development and molecular plasticity in the Mediterranean sea urchin Paracentrotus lividus exposed to CO2-driven acidification. J Exp Biol 214:1357–1368
Melzner F, Gutowska MA, Langenbuch M, Dupont S, Lucassen M, Thorndyke MC, Bleich M, Pörtner HO (2009) Physiological basis for high CO2 tolerance in marine ectothermic animals: pre-adaptation through lifestyle and ontogeny? Biogeosciences 6:2313–2331
Meunier B, Bouley J, Piec I, Bernard C, Picard B, Hocquette JF (2005) Data analysis methods for detection of differential protein expression in two-dimensional gel electrophoresis. Anal Biochem 340:226–230
Mezhoud K, Bauchet AL, Chateau-Joubert S, Praseuth D, Marie A, François JC, Fontaine JJ, Jaeg JP, Cravedi JP, Puiseux-Dao S (2008) Proteomic and phosphoproteomic analysis of cellular responses in medaka fish (Oryzias latipes) following oral gavage with microcystin-LR. Toxicon 51:1431–1439
Miller AW, Reynolds AC, Sobrino C, Riedel GF (2009) Shellfish face uncertain future in high CO2 world: influence of acidification on oyster larvae calcification and growth in estuaries. PLoS ONE 4:1–8
Millero FJ, Graham TB, Huang F, Bustos-Serrano H, Pierrot D (2006) Dissociation constants of carbonic acid in seawater as a function of salinity and temperature. Mar Chem 100:80–94
Mos B, Cowden KL, Nielsen SJ, Dworjanyn SA (2011) Do cues matter? Highly inductive settlement cues don’t ensure high post-settlement survival in sea urchin aquaculture. PLoS ONE 6:e28054
Mumby M, Brekken D (2005) Phosphoproteomics: new insights into cellular signaling. Genome Biol 6:230–238
Nakamura M, Ohki S, Suzuki A, Sakai K (2011) Coral larvae under ocean acidification: survival, metabolism, and metamorphosis. PLoS ONE 6:e14521
O’Connor MI, Bruno JF, Gaines SD, Halpern BS, Lester SE, Kinlan BP, Weiss JM (2007) Temperature control of larval dispersal and the implications for marine ecology, evolution, and conservation. Proc Natl Acad Sci USA 104:1266–1271
O’Donnell MJ, Todgham AE, Sewell MA, Hammond LTM, Ruggiero K, Fangue NA, Zippay ML, Hofmann GE (2010) Ocean acidification alters skeletogenesis and gene expression in larval sea urchins. Mar Ecol Prog Ser 398:157–171
Onitsuka T, Kawamura T, Ohashi S, Iwanaga S, Horii T, Watanabe Y (2010) Effects of delayed metamorphosis and delayed post-settlement feeding on post-larval survival and growth of the abalone Haliotis diversicolor. Aquaculture 298:239–244
Parker LM, Ross PM, O’Connor WA (2009) The effect of ocean acidification and temperature on the fertilization and embryonic development of the Sydney rock oyster Saccostrea glomerata (Gould 1850). Glob Change Biol 15:2123–2136
Parker L, Ross P, O’Connor W (2010) Comparing the effect of elevated pCO2 and temperature on the fertilization and early development of two species of oysters. Mar Biol 157:2435–2452
Parker L, Ross P, O’Connor W (2011) Populations of the Sydney rock oyster, Saccostrea glomerata, vary in response to ocean acidification. Mar Biol 158:689–697
Parker LM, Ross PM, O’Connor WA, Borysko L, Raftos DA, Pörtner HO (2012) Adult exposure influences offspring response to ocean acidification in oysters. Glob Change Biol 18:82–92
Pechenik JA (1999) On the advantages and disadvantages of larval stages in benthic marine invertebrates life cycles. Mar Ecol Prog Ser 177:269–297
Pelletier G, Lewis E, Wallace D (2005) A calculator for the CO2 system in seawater for Microsoft Excel/VBA. Washington State Department of Ecology, Olympia, WA, Brookhaven National Laboratory, Upton, NY
Pörtner HO, Dupont S, Melzner F, Storch D, Thorndyke M (2010) Studies of metabolic rate and other characters across life stages. In: Riebesell U, Fabry VJ, Hansson L, Gattuso JP (eds) Guide to best practices for ocean acidification research and data reporting. Publications Office of the European Union, Luxembourg
Rabilloud T, Lelong C (2011) Two-dimensional gel electrophoresis in proteomics: a tutorial. J Proteomics 74:1829–1841
Ramagli LS (1999) Quantifying protein in 2-D PAGE solubilization buffers. Methods Mol Biol 112:99–104
Rico-Villa B, Le Coz JR, Mingant C, Robert R (2006) Influence of phytoplankton diet mixtures on microalgae consumption, larval development and settlement of the Pacific oyster Crassostrea gigas (Thunberg). Aquaculture 256:377–388
Rico-Villa B, Pouvreau S, Robert R (2009) Influence of food density and temperature on ingestion, growth and settlement of Pacific oyster larvae, Crassostrea gigas. Aquaculture 287:395–401
Rico-Villa B, Bernard I, Robert R, Pouvreau S (2010) A Dynamic Energy Budget (DEB) growth model for Pacific oyster larvae, Crassostrea gigas. Aquaculture 305:84–94
Riebesell U, Fabry VJ, Hansson L, Gattuso JP (2010) Guide to best practices for ocean acidification research and data reporting. Publications Office of the European Union
Rodrigues PM, Silva TS, Dias J, Jessen F (2012) Proteomics in aquaculture: applications and trends. J Proteomics 75:4325–4345
Ross PM, Parker L, O’Connor WA, Bailey EA (2011) The impact of ocean acidification on reproduction, early development and settlement of marine organisms. Water 3:1005–1030
Saeed AI, Bhagabati NK, Braisted JC, Liang W, Sharov V, Howe EA, Li J, Thiagarajan M, White JA, Quackenbush J (2006) TM4 Microarray software suite. Methods Enzymol 411:134–193
Sanchez BC, Ralston-Hooper K, Sepúlveda MS (2011) Review of recent proteomic applications in aquatic toxicology. Environ Toxicol Chem 30:274–282
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Shevchenko A, Wilm M, Vorm O, Mann M (1996) Mass spectrometric sequencing of proteins from silver-stained polyacrylamide gels. Anal Chem 68:850–858
Slattery M, Ankisetty S, Corrales J, Marsh-Hunkin KE, Gochfeld DJ, Willett KL, Rimoldi JM (2012) Marine proteomics: a critical assessment of an emerging technology. J Nat Prod doi:10.1021/np300366a
Stasyk T, Morandell S, Bakry R, Feuerstein I, Huck CW, Stecher G, Bonn GK, Huber LA (2005) Quantitative detection of phosphoproteins by combination of two-dimensional difference gel electrophoresis and phosphospecific fluorescent staining. Electrophoresis 26:2850–2854. doi:10.1002/elps.200500026
Stumpp M, Dupont S, Thorndyke M, Melzner F (2011) CO2 induced acidification impacts sea urchin larval development II: gene expression patterns in pluteus larvae. Comp Biochem Physiol Part A 160:320–330
Sun J, Wang M, Wang H, Zhang H, Zhang X, Thiyagarajan V, Qian PY, Qiu JW (2012) De novo assembly of the transcriptome of an invasive snail and its multiple ecological applications. Mol Ecol Resour 12:1133–1144. doi:10.1111/1755-0998.12014
Suwa R, Nakamura M, Morita M, Shimada K, Iguchi A, Sakai K, Suzuki A (2010) Effects of acidified seawater on early life stages of scleractinian corals (Genus Acropora). Fish Sci 76:93–99
Talmage SC, Gobler CJ (2009) The effects of elevated carbon dioxide concentrations on the metamorphosis, size, and survival of larval hard clams (Mercenaria mercenaria), bay scallops (Argopecten irradians), and Eastern oysters (Crassostrea virginica). Limnol Oceanogr 54:2072–2080
Talmage SC, Gobler CJ (2010) Effects of past, present, and future ocean carbon dioxide concentrations on the growth and survival of larval shellfish. Proc Natl Acad Sci USA 107:17246–17251
Tamburri MN, Zimmer-Faust RK, Tamplin ML (1992) Natural sources and properties of chemical inducers mediating settlement of oyster larvae: a re-examination. Biol Bull 183:327–338
Thiyagarajan V (2010) A review on the role of chemical cues in habitat selection by barnacles: new insights from larval proteomics. J Exp Mar Biol Ecol 392:22–36
Thiyagarajan V, Ko GWK (2012) Larval growth response of the Portuguese oyster (Crassostrea angulata) to multiple climate change stressors. Aquaculture 370–371:90–95
Thiyagarajan V, Qian PY (2008) Proteomic analysis of larvae during development, settlement, and metamorphosis in the fouling barnacle, Balanus amphitrite. Proteomics 8:3164–3172
Thiyagarajan V, Pechenik JA, Gosselin LA, Qian PY (2007) Juvenile growth in barnacles: combined effect of delayed metamorphosis and sub-lethal exposure of cyprids to low-salinity stress. Mar Ecol Prog Ser 344:173–184
Thiyagarajan V, Wong T, Qian PY (2009) 2D Gel-based proteome and phosphoproteome analysis during larval metamorphosis in two major marine biofouling Invertebrates. J Proteome Res 8:2708–2719
Timmins-Schiffman E, O’Donnell M, Friedman C, Roberts S (2012) Elevated pCO2 causes developmental delay in early larval Pacific oysters, Crassostrea gigas. Mar Biol 1–10. doi:10.1007/s00227-012-2055-x
Todgham AE, Hofmann GE (2009) Transcriptomic response of sea urchin larvae Strongylocentrotus purpuratus to CO2-driven seawater acidification. J Exp Biol 212:2579–2594
Tomanek L (2006) Pitfall or promise: proteomics for non-model organisms. J Exp Biol 209:3
Tomanek L (2011) Environmental proteomics: changes in the proteome of marine organisms in response to environmental stress, pollutants, infection, symbiosis, and development. Ann Rev Mar Sci 3:373–399
Tomanek L, Zuzow MJ, Ivanina AV, Beniash E, Sokolova IM (2011) Proteomic response to elevated pCO2 level in eastern oysters, Crassostrea virginica: evidence for oxidative stress. J Exp Biol 214:1836–1844
Turse JE, Marshall MJ, Fredrickson JK, Lipton MS, Callister SJ (2010) An empirical strategy for characterizing bacterial proteomes across species in the absence of genomic sequences. PLoS ONE 5:e13968
Van Colen C, Debusschere E, Braeckman U, Van Gansbeke D, Vincx M (2012) The early life history of the clam Macoma balthica in a high CO2 world. PLoS ONE 7:e44655
Wang X, Li X, Li Y (2007) A modified Coomassie Brilliant Blue staining method at nanogram sensitivity compatible with proteomic analysis. Biotechnol Lett 29:1599–1603
Watson SA, Southgate PC, Tyler PA, Peck LS (2009) Early larval development of the Sydney rock oyster Saccostrea glomerata under near-future predictions of CO2-driven ocean acidification. J Shellfish Res 28:431–437
Weiss IM, Tuross N, Addadi L, Weiner S (2002) Mollusc larval shell formation: amorphous calcium carbonate is a precursor phase for aragonite. J Exp Zoo Part A 293:478–491
Welladsen HM, Southgate PC, Heimann K (2010) The effects of exposure to near-future levels of ocean acidification on shell characteristics of Pinctada fucata (Bivalvia: Pteriidae). Molluscan Res 30:125–130
Wong KKW, Lane AC, Leung PTY, Thiyagarajan V (2011) Response of larval barnacle proteome to CO2-driven seawater acidification. Comp Bioch Physiol Part D 6:310–321
Yan Z, Fang Z, Ma Z, Deng J, Li S, Xie L, Zhang R (2007) Biomineralization: functions of calmodulin-like protein in the shell formation of pearl oyster. Biochim Biophys Acta 1770:1338–1344
Zar JH (1999) Biostatistical analysis. Prentice Hall, Upper Saddle River
Zeebe RE, Zachos JC, Caldeira K, Tyrrell T (2008) Carbon emissions and acidification. Science 321:51–52
Zhang Y, Sun J, Xiao K, Arellano SM, Thiyagarajan V, Qian PY (2010) 2D Gel-based multiplexed proteomic analysis during larval development and metamorphosis of the biofouling polychaete tubeworm Hydroides elegans. J Proteome Res 9:4851–4860
Acknowledgments
We are grateful to PY Qian (HKUST, Hong Kong), Gray Williams and Kenneth Leung (The University of Hong Kong, Hong Kong) for their constant support during the course of this project. Authors also thank Sam Dupont (University of Gothenburg) and Hans-Otto Pörtner (Alfred Wegener Institute) for their critical editorial comments and efforts to coordinate this special issue on ocean acidification. We thank Mr. Fu for his technical assistance with the experimental setup at the hatchery. The oyster larval culture facilities located in Zhanjiang (China) hatchery were partially funded by the 973 program to Ziniu Yu. This study was primarily supported by a research grant from the HKSAR-RGC (No.778309 M) and the Area of Excellency project (No. AoE/P-04/2004).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Dupont.
Rights and permissions
About this article
Cite this article
Dineshram, R., Thiyagarajan, V., Lane, A. et al. Elevated CO2 alters larval proteome and its phosphorylation status in the commercial oyster, Crassostrea hongkongensis . Mar Biol 160, 2189–2205 (2013). https://doi.org/10.1007/s00227-013-2176-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-013-2176-x