Abstract
Differences in stress tolerance and reproductive traits may drive the competitive hierarchy between non-indigenous and indigenous species and turn the former ones into successful invaders. In the northern Baltic Sea, the non-indigenous Gammarus tigrinus is a recent invader of littoral ecosystems and now occupies comparable ecological niches as the indigenous G. zaddachi. In laboratory experiments on specimens collected between June and August 2009 around Tvärminne in southern Finland (59°50′N/23°15′E), the tolerances towards heat stress and hypoxia were determined for the two species using lethal time, LT50, as response variable. The brood size of the two species was also studied and some observations were made on maturation of juveniles. Gammarus tigrinus was more resistant to hypoxia and survived at higher temperatures than G. zaddachi. Brood size was also greater in G. tigrinus than in G. zaddachi and G. tigrinus matured at a smaller size and earlier than G. zaddachi. Hence, there are clear competitive advantages for the non-indigenous G. tigrinus compared to the indigenous G. zaddachi, and these may be further strengthened through ongoing environmental changes related to increased eutrophication and a warming climate in the Baltic Sea region.



Similar content being viewed by others
References
BACC Author Team (2008) Assessment of climate change for the Baltic Sea basin. Regional Climate Studies. Springer, Berlin
Bacela K, Konopacka A (2005) The life history of Pontogammarus robustoides, an alien amphipod in Polish waters. J Crustacean Biol 25:190–195
Bacela K, Konopacka A, Grabowski M (2009) Reproductive biology of Dikerogammarus haemobaphes: an invasive gammarid (Crustacea: Amphipoda) colonizing running waters in Central Europe. Biol Invasions 11:2055–2066
Berezina N (2007) Expansion of the North American amphipod Gammarus tigrinus Sexton, 1939 to the Neva Estuary (easternmost Baltic Sea). Oceanologia 49:129–135
Bonsdorff E, Pearson TH (1999) Variation in the sublittoral macrobenthos of the Baltic Sea along environmental gradients: a functional group approach. Aust J Ecol 24:312–326
Bousfield EL (1958) Freshwater amphipod crustaceans of glaciated North America. Can Field Nat 72:55–113
Bousfield EL (1973) Shallow-water gammaridean Amphipoda of New England. Cornell University Press, Ithaca
Bownes SJ, McQuaid CD (2009) Mechanisms of habitat segregation between an invasive and an indigenous mussel: settlement, post-settlement mortality and recruitment. Mar Biol 156:991–1006
Bownes SJ, McQuaid CD (2010) Mechanisms of habitat segregation between an invasive (Mytilus galloprovincialis) and an indigenous (Perna perna) mussel: adult growth and mortality. Mar Biol 157:1799–1810
Braby CE, Somero GN (2006) Following the heart: temperature and salinity effects on heart rate in native and invasive species of blue mussels (genus Mytilus). J Exp Biol 209:2554–2566
Bruno JF, Stachowicz JJ, Bertness MD (2003) Inclusion of facilitation into ecological theory. Trends Ecol Evol 18:119–125
Bulnheim H-P (1976) Gammarus tigrinus, ein neues Faunenelement der Ostseeförde Schlei. Schriften des Naturwissenschaftlichen Vereins für Schleswig-Holstein 46:79–84
Christie H, Kraufvelin P (2004) Mechanisms regulating amphipod population density within macroalgal communities with restricted predator impact. Sci Mar 68(Suppl 1):189–198
Daunys D, Zettler ML (2006) Invasion of the North American amphipod (Gammarus tigrinus Sexton 1939) into the Curonian lagoon South-eastern Baltic Sea. Acta Zool Lit 16:20–26
Dick JTA (1996) Post-invasion amphipod communities of Lough Neagh, Northern Ireland: influences of habitat selection and mutual predation. J Anim Ecol 65:756–767
Fenchel TM, Kolding S (1979) Habitat selection and distribution patterns of five species of the amphipod genus Gammarus. Oikos 33:316–322
Ganning B (1971) On the ecology of Heterocypris salinus, H. incongruens and Cypridopsis aculeata (Crustacea : Ostracoda) from Baltic brackish-water rockpools. Mar Biol 8:271–279
Grabowski M, Konopacka A, Jazdzewski K, Janowska E (2006) Invasions of alien gammarid species and retreat of natives in the Vistula Lagoon (Baltic Sea Poland). Helgoland Mar Res 60:90–97
Grabowski M, Bacela K, Konopacka A (2007a) How to be an invasive gammarid (Amphipoda, Gammaroidea)–comparison of life history traits. Hydrobiologia 590:75–84
Grabowski M, Jazdzewski K, Konopacka A (2007b) Alien Crustacea in Polish waters–Amphipoda. Aquat Invasions 3:25–38
Gruszka P (1999) The River Odra estuary as a gateway for alien species immigration to the Baltic Sea basin. Acta Hydroch Hydrob 27:374–382
Herkül K, Kotta J (2007) New records of the amphipods Chelicorophium curvispinum, Gammarus tigrinus, G. duebeni, and G. lacustris in the Estonian coastal sea. Proc Est Acad Sci Biol Ecol 56:290–296
Jazdzewski K, Konopacka A, Grabowski M (2002) Four Ponto-Caspian and one American gammarid species (Crustacea, Amphipoda) recently invading Polish waters. Contrib Zool 71:115–122
Jewett EB, Hines AH, Ruiz GM (2005) Epifaunal disturbance by periodic low levels of dissolved oxygen: native vs. invasive species response. Mar Ecol Prog Ser 304:31–44
Kercher SM, Zedler JB (2004) Flood tolerance in wetland angiosperms: a comparison of invasive and noninvasive species. Aquat Bot 80:89–102
Kinne O (1961) Growth, molting frequency, heart beat, number of eggs, and incubation time in Gammarus zaddachi exposed to different environments. Crustaceana 2:26–34
Köhn J, Gosselck F (1989) Bestimmungschlüssel der Malakostraken der Ostsee. Mitteilungen Zoologisches Museum Berlin 65:3–114
Kolding S, Fenchel TM (1981) Patterns of reproduction in different populations of 5 species of the amphipod genus Gammarus. Oikos 37:167–172
Korpinen S, Westerbom M (2010) Microhabitat segregation of the amphipod genus Gammarus (Crustacea: Amphipoda) in the Northern Baltic Sea. Mar Biol 157:361–370
Kraufvelin P, Salovius S, Christie H, Moy FE, Karez R, Pedersen MF (2006) Eutrophication-induced changes in benthic algae affect the behaviour and fitness of the marine amphipod Gammarus locusta. Aquat Bot 84:199–209
Lee CE (2002) Evolutionary genetics of invasive species. Trends Ecol Evol 17:386–391
Lenz M, da Gama BAP, Gerner NV, Gobin J, Gröner F, Harry A, Jenkins SR, Kraufvelin P, Mummelthei C, Sareyka J, Xavier EA, Wahl M (2011) Non-native marine invertebrates are more tolerant towards environmental stress than taxonomically related native species? Results from a globally replicated study. Environ Res (in press)
Lincoln RJ (1979) British marine Amphipoda: Gammaridea. British Museum(Natural History), London, p 658
Lodge DM (1993) Biological invasions–lessons for ecology. Trends Ecol Evol 8:133–137
Lyons DA, Scheibling RE (2009) Range expansions by invasive marine algae: rates and patterns of spread at a regional scale. Divers Distrib 15:762–775
Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10:689–710
MacNeil C, Platvoet D, Dick JTA (2008) Potential roles for differential body size and microhabitat complexity in mediating biotic interactions within freshwater amphipod assemblages. Fund Appl Limnol 172:175–182
McMahon RF (2002) Evolutionary and physiological adaptations of aquatic invasive animals: r selection versus resistance. Can J Fish Aquat Sci 59:1235–1244
Mooney HA, Cleland EE (2001) The evolutionary impact of invasive species. Proc Natl Acad Sci USA 98:5446–5451
Norkko A, Bonsdorff E (1996) Rapid zoobenthic community responses to accumulations of drifting algae. Mar Ecol Prog Ser 131:143–157
Occhipinti-Ambrogi A, Savini D (2003) Biological invasions as a component of global change in stressed marine ecosystems. Mar Pollut Bull 46:542–551
Oksama M, Kristoffersson R (1979) The toxicity of phenol to Phoxinus phoxinus, Gammarus duebeni and Mesidothea entomon in brackish water. Ann Zool Fenn 16:209–216
Orav-Kotta H, Kotta J, Herkül K, Kotta I, Paalme T (2009) Seasonal variability in the grazing potential of the invasive amphipod Gammarus tigrinus and the native amphipod Gammarus salinus (Amphipoda: Crustacea) in the northern Baltic Sea. Biol Invasions 11:597–608
Paavola M, Olenin S, Leppäkoski E (2005) Are invasive species most successful in habitats of low native species richness across European brackish water seas? Estuar Coast Shelf Sci 64:738–750
Paavola M, Laine AO, Helavuori M, Kraufvelin P (2008) Profiling four brackish water harbours: zoobenthic composition and invasion status. Boreal Environ Res 13:159–175
Packalén A, Korpinen S, Lehtonen KK (2008) The invasive amphipod species Gammarus tigrinus (Sexton 1939) can rapidly change littoral communities in the Gulf of Finland (Baltic Sea). Aquat Invasions 3:405–412
Peterson MS, Slack WT, Brown-Peterson NJ, McDonald JL (2004) Reproduction in non-native environments: Establishment of Nile Tilapia, Oreochromis niloticus, in coastal Mississippi watersheds. Copeia 4:842–849
Pienimäki M, Helavuori M, Leppäkoski E (2004) First finding of the North American amphipod Gammarus tigrinus Sexton, 1939 along the Finnish coast. Mem Soc Fauna Flora Fenn 80:17–19
Pinkster S, Smit H, Brandse-de Jong N (1977) The introduction of the alien amphipod Gammarus tigrinus Sexton, 1939 in the Netherlands and its competition with indigenous species. Crustaceana Suppl 4:91–105
Platvoet D, Dick JTA, MacNeil C, van Riel MC, van der Velde G (2009) Invader-invader interactions in relation to environmental heterogeneity leads to zonation of two invasive amphipods, Dikerogammarus villosus (Sowinsky) and Gammarus tigrinus Sexton: amphipod pilot species project (AMPIS) report 6. Biol Invasions 11:2085–2093
Pöckl M (2009) Success of the invasive Ponto-Caspian amphipod Dikerogammarus villosus by life history traits and reproductive capacity. Biol Invasions 11:2021–2041
Richard J, Huet M, Thouzeau G, Paulet YM (2006) Reproduction of the invasive slipper limpet, Crepidula fornicata, in the Bay of Brest, France. Mar Biol 149:789–801
Ruiz GM, Fofonoff PW, Carlton JT, Wonham MJ, Hines AH (2000) Invasion of coastal marine communities in North America: apparent patterns, processes, and biases. Ann Rev Ecol Syst 31:481–531
Salovius S, Kraufvelin P (2004) The filamentous green alga Cladophora glomerata as a habitat for littoral macrofauna in the northern Baltic Sea. Ophelia 58:65–78
Schmitz W (1960) Die Einbürgerung von Gammarus tigrinus Sexton auf dem europäischen Kontinent. Arch Hydrobiol 57:223–225
Schneider KR, Helmuth B (2007) Spatial variability in habitat temperature may drive patterns of selection between an invasive and native mussel species. Mar Ecol Prog Ser 339:157–167
Segerstråle SG (1957) Baltic Sea. In: Hedgpeth JW (ed) Treatise on marine ecology and paleoecology I, vol 67. Ecology, Geological Society of America, Washington DC, pp 751–800
Stachowicz JJ, Byrnes JE (2006) Species diversity, invasion success, and ecosystem functioning: disentangling the influence of resource competition, facilitation, and extrinsic factors. Mar Ecol Prog Ser 311:251–262
Szaniawska A, Lapucki T, Normant M (2003) The invasive amphipod Gammarus tigrinus Sexton, 1939, in Puck Bay. Oceanologia 45:507–510
Szaniawska A, Normant M, Lapucki T (2005) Gammarus tigrinus Sexton 1939 (Crustacea, Amphipoda) a new immigrant in the Puck Bay, southern Baltic Sea. Oceanol Hydrobiol St 34:71–78
Thomsen MS, McGlathery KJ (2007) Stress tolerance of the invasive macroalgae Codium fragile and Gracilaria vermiculophylla in a soft-bottom turbid lagoon. Biol Invasions 9:499–513
Vahteri P, Mäkinen A, Salovius S, Vuorinen I (2000) Are drifting algal mats conquering the bottom of the Archipelago Sea, SW Finland? Ambio 29:338–343
Voipio A (1981) The Baltic Sea. Elsevier oceanography series 30. Elsevier, Amsterdam, p 418
Wijnhoven S, van Riel MC, van der Velde G (2003) Exotic and indigenous freshwater gammarid species: physiological tolerance to water temperature in relation to ionic content of the water. Aquat Ecol 37:151–158
Williamson MH, Fitter A (1996) The characters of successful invaders. Biol Conserv 78:163–170
Zardi GI, Nicastro KR, McQuaid CD, Erlandsson J (2008) Sand and wave induced mortality in invasive (Mytilus galloprovincialis) and indigenous (Perna perna) mussels. Mar Biol 153:853–858
Zettler ML (1995) Ertsnachweis von Gammarus tigrinus Sexton, 1939 (Crustacea: Amphipoda) in der Darst-Zingster-Boddenkette und seine derzeitige Verbreitung an der deutschen Ostseeküste. Archiv der Freunde der Naturgeschichte in Mecklenburg 34:137–140
Acknowledgments
This study was part of the international research and training programme GAME VII (Global Approach by Modular Experiments) which is generously funded by the Mercator Foundation (Essen, Germany) and the Foundation of Kiel University. Financial support was also received from the Walter and Andrée de Nottbeck Foundation (JS) and from Societas pro Fauna et Flora Fennica (PK). We are grateful to the staff at Tvärminne Zoological Station for providing excellent working facilities for the project and for kind hospitality to JS. Three anonymous reviewers provided many insightful comments that greatly improved the paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. P. Grassle.
Appendix
Appendix
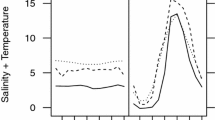
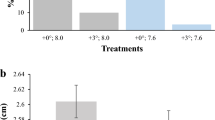
See Fig. 3.
Rights and permissions
About this article
Cite this article
Sareyka, J., Kraufvelin, P., Lenz, M. et al. Differences in stress tolerance and brood size between a non-indigenous and an indigenous gammarid in the northern Baltic Sea. Mar Biol 158, 2001–2008 (2011). https://doi.org/10.1007/s00227-011-1708-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-011-1708-5