Abstract
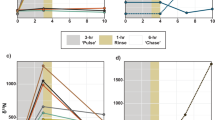
We quantified the nitrogen and enzyme hydrolyzable amino acid (EHAA) concentrations of sediments prior to and after corals sloughed, ingested, and egested sediments layered onto their surfaces, for the three coral species Siderastrea siderea, Agaricia agaricites, and Porites astreoides in Jamaica. The percent nitrogen of the sediments egested by all three species was lower than in the sediments available to the corals. Additionally, the sediments sloughed (not ingested) by A. agaricites and P. astreoides were lower in percent nitrogen, while the sediments sloughed by S. siderea had the same percent nitrogen as that of the available sediments. The percent nitrogen of the sediments sloughed and egested by P. astreoides showed significant negative and positive relationships, respectively, to increasing sediment loads, while the percent nitrogen of the sediments sloughed and egested by both S. siderea and A. agaricites showed no relationship to sediment load. EHAA concentrations were not significantly different between the sloughed and available sediments but were significantly lower in the sediments egested by S. siderea and A. agaricites (EHAA concentrations were not measured for P. astreodies sediment fractions). Comparisons of the nitrogen and EHAA concentrations in the sloughed and egested sediments to what was available prior to coral processing show that maximum ingestion was between 0.1 and 0.2 µg N µg−1 coral N cm−2 and between 0.5 and 0.6 µg EHAA·cm−2. Maximum assimilation efficiencies were estimated to be 30–60% of the available nitrogen. The data show that corals ingest and alter the nitrogen concentration of particles that land on their surfaces. The corals’ abilities to process these sediments, and the sediments’ possible contributions to coral nutrition, are discussed based on these results.






Similar content being viewed by others
References
Alongi DM (1990) Abundances of benthic microfauna in relation to outwelling of mangrove detritus in a tropical coastal region. Mar Ecol Prog Ser 63:53–63
Anthony KRN (1999a) Coral suspension feeding on fine particulate matter. J Exp Mar Biol Ecol 232:85–106
Anthony KRN (1999b) A tank system for studying benthic aquatic organisms at predictable levels of turbidity and sedimentation: case study examining coral growth. Limnol Oceanogr 44:1415–1422
Anthony KRN (2000) Enhanced particle-feeding capacity of nearshore reef corals on the Great Barrier Reef, Australia. Coral Reefs 19:59–67
Anthony KRN, Fabricius KE (2000) Shifting roles of heterotrophy and autotrophy in coral energetics under varying turbidity. J Exp Mar Biol Ecol 252:221–253
Bailey TG, Robertson DR (1982) Organic and caloric levels of fish feces relative to its consumption by coprophagus reef fishes. Mar Biol 69:45–50
Bak RPM (1976) The growth of coral colonies and the importance of crustose coralline algae and burrowing sponges in relation with carbonate accumulation. Neth J Sea Res 10:285–337
Bak RPM, Joenje M, Jong ID, Lambrechts DYM, Nieuwland G (1998) Bacterial suspension feeding by coral reef benthic organisms. Mar Ecol Prog Ser 175:285–288
Capone DG, Dunham SE, Horrigan SG, Duguay LE (1992) Microbial nitrogen transformation in unconsolidated reef sediments. Mar Ecol Prog Ser 80:75–88
Coffroth MA (1990) Mucous sheet formation on poritid corals: an evaluation of coral mucus as a nutrient source on reefs. Mar Biol 105:39–49
Coma R, Ribes M, Gili JM, Hughes RN (2001) The ultimate opportunists: consumers of seston. Mar Ecol Prog Ser 219:305–308
Crossman JC, Choat JH, Clements KD, Hardy T, McConchie J (2001) Detritus as food for grazing fishes on coral reefs. Limnol Oceanogr 46:1596–1605
Dodge RE, Vaisnys JR (1977) Coral populations and growth patterns: response to sedimentation and turbidity associated with dredging. J Mar Res 35:715–730
Dommisse M (2001) The potential nutritional value of detritus depositing onto coral reefs. James Cooke University, Townsville, Australia
Ducklow HW, Mitchell R (1979) Composition of mucus released by coral reef coelenterates. Limnol Oceanogr 24:706–714
Edmunds PJ, Davies PS (1986) An energy budget for Porites porites (Scleractinia). Mar Biol 92:339–347
Fabricius KE, Dommisse M (2000) Depletion of suspended particulate matter over coastal reef communities dominated by zooxanthellate soft coral. Limnol Oceanogr 196:157–167
Fabricius KE, Benayahu Y, Genin A (1995) Herbivory in asymbiotic soft corals. Science 268:90–92
Ferrier MD (1991) Net uptake of dissolved free amino acids by four scleractinian corals. Coral Reefs 10:183–187
Findlay S, Tenore KR (1982) Nitrogen source for a detritivore: detritus substrate vs. associated microbes. Science 218:371–373
Gast GJ, Wiegman S, Wieringa E, Duyl FC van, Bak RPM (1998) Bacteria in coral reef water types: removal of cells, stimulation of growth and mineralization. Mar Ecol Prog Ser 167:37–45
Larcombe P, Ridd PV, Wilson B, Prytz A (1995) Factors controlling suspended sediment on inner-shelf coral reefs, Townsville, Australia. Coral Reefs 14:163–171
Lewis JB, Price WS (1975) Feeding mechanisms and feeding strategies of Atlantic reef corals. J Zool (Lond) 176:527–544
Lewis JB, Price WS (1976) Patterns of ciliary currents in Atlantic reef corals and their functional significance. J Zool (Lond) 178:77–89
Lopez GR, Levinton JS (1987) Ecology of marine deposit-feeding animals in marine sediments. Q Rev Biol 62:235–260
Marsh JA (1970) Primary productivity of reef-building calcareous red algae. Ecology 51:255–263
Mayer LM, Schick LL, Setchell FW (1986) Measurement of protein in nearshore marine sediments. Mar Ecol Prog Ser 30:159–165
Mayer LM, Schick LL, Sawyer T, Plante CJ, Jumars PA, Self RL (1995) Bioavailable amino acids in sediments: a biomimetic, kinetics-based approach. Limnol Oceanogr 40:511–520
Means JC, Sigleo AC (1986) Contribution of coral reef mucus to the colloidal organic pool in the vicinity of Discovery Bay, Jamaica, W.I. Bull Mar Sci 39:110–118
Meikle P, Richards GN, Yellowlees D (1988) Structural investigations on the mucus from six species of coral. Mar Biol 99:187–193
Meyer JL, Schultz ET (1985) Migrating haemulid fishes as a source of nutrition and organic matter on coral reefs. Limnol Oceanogr 30:146–156
Mills MM, Sebens KP (1997) Particle ingestion efficiency of the corals Siderastrea siderea and Agaricia agaricites: effects of flow speed and sediment loads. Proc 8th Int Coral Reef Symp 2:1059–1064
Mills MM, Lipschultz F, Sebens KP (2004) Particulate matter ingestion and associated nitrogen uptake by four species of scleractinian corals. Coral Reefs (in press)
Nixon SW, Pilson EQ (1983) Nitrogen in estuarine and marine coastal ecosystems. In: Carpenter EJ, Capone DG (ed) Nitrogen in the marine environment. Academic Press, New York, pp 565–648
Ribes M, Coma R, Gili JM (1998) Heterotrophic feeding by gorgonian corals with symbiotic zooxanthellae. Limnol Oceanogr 43:1170–1179
Rice DL (1982) The detritus nitrogen problem: new observations and perspectives from organic geochemistry. Mar Ecol Prog Ser 9:153–162
Robertson DR (1982) Fish feces as food on a Pacific coral reef. Mar Ecol Prog Ser 7:253–265
Rogers CS (1990) Responses of coral reefs and reef organisms to sedimentation. Mar Ecol Prog Ser 62:185–202
Rothans TC, Miller AC (1991) A link between biologically imported particulate organic nutrients and the detritus food web in reef communities. Mar Biol 110:145–150
Schaffelke B (1999) Particulate organic matter as an alternative nutrient source for tropical Sargassum species (Fucales, Phaeophyceae). J Phycol 35:1158–1161
Sebens KP, Grace SP, Helmuth B, Maney EJ, Miles JS (1998) Water flow and prey capture by three scleractinian coral, Madracis mirabilis, Montastrea cavernosa, and Porites porites, in a field enclosure. Mar Biol 131:347–360
Szmant AM, Ferrer LM, Fitzgerald LM (1990) Nitrogen excretion and O:N ratios in reef corals: evidence for conservation of nitrogen. Mar Biol 104:119–127
Tomascik T, Sander F (1985) Effects of eutrophication on reef-building corals I. Growth rate of the reef-building coral Montastrea annularis. Mar Biol 87:143–155
Widdig A, Schlichter D (2001) Phytoplankton: a significant trophic source for soft corals? Helgol Mar Res 55:198–211
Williams SL, Gill IP, Yarish SM (1985) Nitrogen cycle in back reef sediments. Proc 5th Int Coral Reef Symp 3:389–394
Yahel R, Yahel G, Genin A (2002) Daily cycles of suspended sand at coral reefs: a biological control. Limnol Oceanogr 47:1071–1083
Yahel G, Sharp JH, Häse C, Genin A (2003) In situ feeding and element removal in the symbiont-bearing sponge Theonella swinhoei: bulk DOC is the major source for carbon. Limnol Oceanogr 48:141–149
Acknowledgements
We thank L. Wooton and B. McGee for help with the EHAA protocol and analysis, and L. Lane from the Horn Point Laboratory’s Analytical Services, who analyzed the nitrogen samples. Much appreciated field assistance was provided by K. Heidelberg, S. P. Grace, and K. S. Vandersall. We are also grateful to the Discovery Bay Marine Laboratory (University of the West Indies) for providing laboratory space and equipment. We also thank G. M. Berg for providing helpful comments on the manuscript. This project was supported by NSF grant OCE9302066 to K.P. Sebens.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by O. Kinne, Oldendorf/Luhe
Rights and permissions
About this article
Cite this article
Mills, M.M., Sebens, K.P. Ingestion and assimilation of nitrogen from benthic sediments by three species of coral. Marine Biology 145, 1097–1106 (2004). https://doi.org/10.1007/s00227-004-1398-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-004-1398-3