Abstract
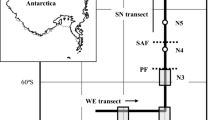
The pelagic nekton community was sampled with the RMT 25 opening/closing net and a neuston net at two stations in the Scotia Sea south of the Antarctic Polar Front in the open ocean (Station 1) and on the South Georgia northwestern slope (Station 2). Downward oblique tows were made with the RMT 25 through discrete 200 m layers to 1000 m in daylight and darkness. A total of 119 cephalopods representing nine species were removed from the samples, and mantle and arm lengths were measured to the nearest 0.1 mm. The most abundant species at each station was an undescribed Brachioteuthis sp. (B. ?picta). Galiteuthis glacialis and Alluroteuthis antarcticus were caught at both stations. Histioteuthis eltaninae, Bathyteuthis abyssicola and Psychroteuthis glacialis were caught at Station 1. Mastigoteuthis psychrophila and a Chiroteuthis sp. were caught at Station 2. B. ?picta was present throughout the water column to 1000 m at both stations, with little evidence of ontogenetic descent. There was evidence for ontogenetic descent in G. glacialis. This species was absent from the Antarctic Surface Water (ASW) at Station 1, where it was concentrated in the Circumpolar Deep Water (CDW). At Station 2 it was present throughout the water column to 1000 m. The other species were all caught in the core of the CDW (>400 m). In juvenile B. ?picta, G. glacialis and A. antarcticus, growth of the brachial crown is positively allometric with respect to mantle length. Recent data on biomass spectra in high-latitude pelagic systems show that they are characterised by the presence of peaks of biomass separated by biomass minima. Positive allometric growth in the brachial crown of these antarctic oceanic squid is suggested to have evolved as an adaptation to the peaked, or domed, structure of the pelagic biomass spectrum which must be spanned by these predators as their optimum prey size increases with growth. Interspecific differences in the allometry of tentacle growth are probably related to differences in strategies for stalking and capture of prey.
Similar content being viewed by others
References
Boudreau PR, Dickie LM (1992) Biomass spectra of aquatic ecosystems in relation to fisheries yield. Can J Fish aquat Sciences 49: 1528–1538
Clarke MR (1980) Cephalopods in the diet of sperm whales of the southern hemisphere and their bearing on sperm whale biology. ‘Discovery’ Rep 37: 1–324
Imber MJ (1992) Cephalopods eaten by wandering albatrosses (Diomedea exulans L.) breeding at six circumpolar localities. Jl R Soc NZ 22: 243–263
Lipinski MR, Jackson S (1989) Surface-feeding on cephalopods by procellariiform seabirds in the Southern Benguela region, South Africa. J Zool. Lond 218: 549–563
Nesis KN (1987) Cephalopods of the world. T.F.H. Publications Inc., Neptune City, New Jersey
Offredo C, Ridoux V, Clarke MR (1985) Cephalopods in the diet of Emperor and Adelie penguins in Adelie Land, Antarctica. Mar Biol 86: 199–202
Packard A (1972) Cephalopods and fish: the limits of convergence. Biol Rev 47: 241–307
Piatkowski U, Rodhouse PG, Murphy EJ, White MG, Bone DG (1994) Nekton community of the Scotia Sea as sampled by the RMT 25 during austral summer. Mar Ecol Prog Ser 112: 13–28
Rodhouse PG (1988) Distribution of the neoteuthid squid Alluroteuthis antarcticus Odhner in the Atlantic sector of the Southern Ocean. Malacologia 29: 267–274
Rodhouse PG (1990) Cephalopod fauna of the Scotia Sea at South Georgia: potential for commercial exploitation and possible consequences. In: Kerry K. Hempel G (eds) Ecological change and the conservation of Antarctic ecosystems. Proceedings of the 5th SCAR Symposium on Antarctic Biology. Springer-Verlag, Berlin, pp 289–298
Rodhouse PG, Ambom TR, Fedak MA, Yeatman J, Murray AWA (1992a) Cephalopod prey of the southern elephant seal, Mirounga leonina L. Can J Zool 70: 1007–1015
Rodhouse PG, Clarke MR (1985) Growth and distribution of young Mesonychoteuthis hamiltoni Robson (Mollusca: Cephalopods): an Antarctic squid. Vie Milieu 35: 223–230
Rodhouse PG, Clarke MR (1986) Distribution of the early-life phase of the Antarctic squid Galiteuthis glacialis in relation to the hydrology of the Southern Ocean in the sector 15° to 30°E. Mar Biol 91: 353–357
Rodhouse PG, Clarke MR, Murray AWA (9187) Cephalopod prey of the wandering albatross Diomedea exulans. Mar. Biol 96: 1–10
Rodhouse PG, Piatkowski U, Murphy EJ, White MG, Bone DG (1994) Utility and limits of biomass spectra: the nekton community sampled with the RMT 25 in the Scotia Sea during austral summer. Mar Ecol Prog Ser 112: 29–39
Rodhouse PG, Prince PA (1993) Cephalopod prey of the blackbrowed albatross Diomedea melanophrys at South Georgia. Polar Biol 13: 373–376
Rodhouse PG, Prince PA, Clarke MR, Murray AWA (1990) Cephalopod prey of the grey-headed albatross Diomedea chrysostoma. Mar Biol 104: 353–362
Rodhouse PG, Symon C, Hatfield EMC (1992b) Early life cycle of cephalopods in relation to the major oceanographic features of the southwest Atlantic. Mar Ecol Prog Ser 89: 183–195
Roper CFE (1983) An overview of cephalopod systematics: status, problems and recommendations Mem natn Mus Vict 44: 13–27
Roper CFE, Sweeney MJ, Clarke MR (1985) Cephalopods. In: Fischer W, Hureau JC (eds). FAO species identification sheets for fishery purposes. Southern Ocean (Fishing areas 48, 58 and 88) (CCAMLR Convention Area). Vol. I. Prepared and published with the support of the Commission for the Conservation of Antarctic Living Marine Resources. FAO, Rome, pp 117–205
Ryan BF, Joiner BL, Thomas A, Ryan Jr TA (1985) Minitab handbook. 2nd edn. Duxbury Press, Boston, Mass
Vidal EAG (1994) Relative growth of paralarvae and juveniles of Illex argentinus (Castellanos, 1960) in Southern Brazil. Antarct Sci 6: 275–282
Witek Z, Krajewska-Soltys A (1989) Some examples of the epipelagic plankton size structure in high latitude oceans. J Plankton Res 11: 1143–1155
Author information
Authors and Affiliations
Additional information
Communicated by J. Mauchline, Oban
Rights and permissions
About this article
Cite this article
Rodhouse, P.G., Piatkowski, U. Fine-scale distribution of juvenile cephalopods in the Scotia Sea and adaptive allometry of the brachial crown. Marine Biology 124, 111–117 (1995). https://doi.org/10.1007/BF00349152
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00349152