Abstract
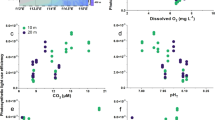
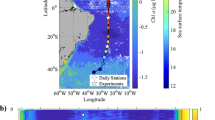
Carbon dioxide and light are two major prerequisites of photosynthesis. Rising CO2 levels in oceanic surface waters in combination with ample light supply are therefore often considered stimulatory to marine primary production1,2,3. Here we show that the combination of an increase in both CO2 and light exposure negatively impacts photosynthesis and growth of marine primary producers. When exposed to CO2 concentrations projected for the end of this century4, natural phytoplankton assemblages of the South China Sea responded with decreased primary production and increased light stress at light intensities representative of the upper surface layer. The phytoplankton community shifted away from diatoms, the dominant phytoplankton group during our field campaigns. To examine the underlying mechanisms of the observed responses, we grew diatoms at different CO2 concentrations and under varying levels (5–100%) of solar radiation experienced by the phytoplankton at different depths of the euphotic zone. Above 22–36% of incident surface irradiance, growth rates in the high-CO2-grown cells were inversely related to light levels and exhibited reduced thresholds at which light becomes inhibitory. Future shoaling of upper-mixed-layer depths will expose phytoplankton to increased mean light intensities5. In combination with rising CO2 levels, this may cause a widespread decline in marine primary production and a community shift away from diatoms, the main algal group that supports higher trophic levels and carbon export in the ocean.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Schippers, P., Lürling, M. & Scheffer, M. Increase of atmospheric CO2 promotes phytoplankton productivity. Ecol. Lett. 7, 446–451 (2004).
Riebesell, U. & Tortell, P. D. in Effects of Ocean Acidification on Pelagic Organisms and Ecosystems in Ocean Acidification (eds Gattuso, J. P. & Hansson, L.) 291–311 (Oxford Univ. Press, 2011).
Hein, M. & Sand-Jensen, K. CO2 increases oceanic primary production. Nature 388, 526–527 (1997).
IPCC Climate Change 2001: The Scientific Basis (eds Houghton, J. T. et. al) (Cambridge Univ. Press, 2001).
Boyd, P. W., Strzepek, R., Fu, F. & Hutchins, D. A. Environmental control of open-ocean phytoplankton groups: Now and in the future. Limnol. Oceanogr. 55, 1353–1376 (2010).
Sabine, C. L. et al. The oceanic sink for anthropogenic CO2 . Science 305, 367–371 (2004).
Caldeira, K. & Wickett, M. E. Oceanography: Anthropogenic carbon and ocean pH. Nature 425, 365 (2003).
Feely, R. A. et al. Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science 305, 362–366 (2004).
Riebesell, U. et al. Reduced calcification of marine plankton in response to increased atmospheric CO2 . Nature 407, 364–367 (2000).
Beaufort, L. et al. Sensitivity of coccolithophores to carbonate chemistry and ocean acidification. Nature 476, 80–83 (2011).
Gao, K. S. & Zheng, Y. Q. Combined effects of ocean acidification and solar ultraviolet radiation on photosynthesis, growth, pigmentation and calcification of the coralline alga Corallina sessilis (Rhodophyta). Glob. Change Biol. 16, 2388–2398 (2010).
Fine, M. & Tchernov, D. Scleractinian coral species survive and recover from decalcification. Science 315, 1811 (2007).
Hutchins, D. et al. CO2 control of Trichodesmium N2 fixation, photosynthesis, growth rates, and elemental ratios: Implications for past, present, and future ocean biogeochemistry. Limnol. Oceanogr. 52, 1293–1304 (2007).
Tortell, P. D., Rau, G. H. & Morel, F. M. M. Inorganic carbon acquisition in coastal Pacific phytoplankton communities. Limnol. Oceanogr. 45, 1485–1500 (2000).
Riebesell, U. et al. Enhanced biological carbon consumption in a high CO2 ocean. Nature 450, 545–548 (2007).
Hutchins, D. A., Mulholland, M. R. & Fu, F. Nutrient cycles and marine microbes in a CO2-enriched ocean. Oceanography 22, 128–145 (2009).
Behrenfeld, M. J. et al. Climate-driven trends in contemporary ocean productivity. Nature 444, 752–755 (2006).
Boyce, D. G., Lewis, M. R. & Worm, B. Global phytoplankton decline over the past century. Nature 466, 591–596 (2010).
Boyd, P. W. Beyond ocean acidification. Nature Geosci. 4, 273–274 (2011).
Nelson, D. M., Tréguer, P., Brzezinski, M. A., Leynaert, A. & Quéguiner, B. Production and dissolution of biogenic silica in the ocean: Revised global estimates, comparison with regional data and relationship to biogenic sedimentation. Glob. Biogeochem. Cycles 9, 359–359 (1995).
Wu, H., Cockshutt, A. M., McCarthy, A. & Campbell, D. A. Distinctive PSII photoinactivation and protein dynamics in marine diatoms. Plant Physiol. 156, 2184–2195 (2011).
Wu, Y., Gao, K. & Riebesell, U. CO2-induced seawater acidification affects physiological performance of the marine diatom Phaeodactylum tricornutum. Biogeosciences 7, 2915–2923 (2010).
Hopkinson, B. M., Dupont, C. L., Allen, A. E. & Morel, F. M. M. Efficiency of the CO2-concentrating mechanism of diatoms. Proc. Natl Acad. Sci. USA 108, 3830–3837 (2011).
Raven, J. A., Giordano, M., Beardall, J. & Maberly, S. C. Algal and aquatic plant carbon concentrating mechanisms in relation to environmental change. Photosynth. Res. 109, 281–296 (2011).
Chen, X. & Gao, K. Characterization of diurnal photosynthetic rhythms in the marine diatom Skeletonema costatum grown in synchronous culture under ambient and elevated CO2 . Funct. Plant Biol. 31, 399–404 (2004).
Rost, B., Riebesell, U., Burkhardt, S. & Sueltemeyer, D. Carbon acquisition of bloom-forming marine phytoplankton. Limnol. Oceanogr. 48, 55–67 (2003).
Wingler, A., Lea, P. J., Quick, W. P. & Leegood, R. C. Photorespiration: Metabolic pathways and their role in stress protection. Phil. Trans. R. Soc. Lond. B. 355, 1517–1529 (2000).
Boyd, P. W. & Doney, S. C. Modelling regional responses by marine pelagic ecosystems to global climate change. Geophys. Res. Lett. 29, 1806 (2002).
Gao, K. S., Ruan, Z. X., Villafane, V. E., Gattuso, J. P. & Helbling, E. W. Ocean acidification exacerbates the effect of ultraviolet radiation on the calcifying phytoplankter Emiliania huxleyi. Limnol. Oceanogr. 54, 1855–1862 (2009).
Chen, S. & Gao, K. Solar ultraviolet radiation and CO2-induced ocean acidification interacts to influence the photosynthetic performance of the red tide alga Phaeocystis globosa (Prymnesiophyceae). Hydrobiologia 675, 105–117 (2011).
Gao, K. et al. Solar ultraviolet radiation drives CO2 fixation in marine phytoplankton: A double-edged sword. Plant Physiol. 144, 54–59 (2007).
Genty, B., Briantais, J. M. & Baker, N. R. The relationship between the quantum yield of photosynthetic electron-transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 990, 87–92 (1989).
Morel, F. M. M., Rueter, J. G., Anderson, D. M. & Guillard, R. R. L. Aquil: A chemically defined phytoplankton culture medium for trace metal studies. J. Phycol. 15, 135–141 (1979).
Guillard, R. R. & Ryther, J. H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can. J. Microbiol. 8, 229–239 (1962).
Acknowledgements
We thank the expedition chief scientists M. Dai, P. Cai and W. Zhai and the crew from Dong-Fang-Hong for their support and help during the cruises. The cruise and laboratory studies were supported by the National Basic Research Program of China (2009CB421207, 2011CB200902) and by the National Natural Science Foundation of China (no. 41120164007 and no. 40930846). The Changjiang Scholars and Innovative Research Team project (IRT0941) and China–Japan collaboration project from the Ministry of Science and Technology (S2012GR0290) are also acknowledged for the field work. D.A.H’s contribution was supported by the United States National Science Foundation Division of Ocean Sciences grants 0942379, 0962309 and 1043748. U.R. acknowledges support by the German Ministry of Education and Research through the project BIOACID. Visits of D.A.H. and U.R. to Xiamen were supported by the 111 project and by the State Key Laboratory of Marine Environmental Science (Xiamen University). The visit of K.G. to Kiel was supported by the German Academic Exchange Service (DAAD).
Author information
Authors and Affiliations
Contributions
On the basis of an original idea from K.G., the concept of this paper was developed in discussion between all authors. J.X. and G.G. contributed as equally as K.G. for their leading roles in laboratory and field experiments, respectively. U.R. and D.A.H. contributed to experimental designs, data analysis and the writing of the paper. D-P.H. contributed to the analysis of the data and writing of the paper. G.G., Y.Z., P.J., K.X., B.H., L.W. and N.L. carried out shipboard experiments; J.X., Y.L., X.C. and W.L. carried out laboratory experiments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Gao, K., Xu, J., Gao, G. et al. Rising CO2 and increased light exposure synergistically reduce marine primary productivity. Nature Clim Change 2, 519–523 (2012). https://doi.org/10.1038/nclimate1507
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nclimate1507
This article is cited by
-
Acidification of seawater attenuates the allelopathic effects of Ulva pertusa on Karenia mikimotoi
Environmental Science and Pollution Research (2023)
-
Enhancement of diatom growth and phytoplankton productivity with reduced O2 availability is moderated by rising CO2
Communications Biology (2022)
-
Effects of spectral light quality on the growth, productivity, and elemental ratios in differently pigmented marine phytoplankton species
Journal of Applied Phycology (2022)
-
Light history modulates growth and photosynthetic responses of a diatom to ocean acidification and UV radiation
Marine Life Science & Technology (2022)
-
Approaches and involved principles to control pH/pCO2 stability in algal cultures
Journal of Applied Phycology (2021)