Abstract
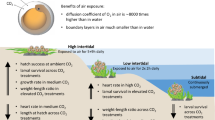
Absorption of anthropogenic carbon dioxide by the world’s oceans is causing mankind’s ‘other CO2 problem’, ocean acidification1. Although this process will challenge marine organisms that synthesize calcareous exoskeletons or shells2,3,4,5,6, it is unclear how it will affect internally calcifying organisms, such as marine fish7. Adult fish tolerate short-term exposures to CO2 levels that exceed those predicted for the next 300 years (∼2,000 ppm; ref. 8), but potential effects of increased CO2 on growth and survival during the early life stages of fish remain poorly understood7. Here we show that the exposure of early life stages of a common estuarine fish (Menidia beryllina) to CO2 concentrations expected in the world’s oceans later this century caused severely reduced survival and growth rates. When compared with present-day CO2 levels (∼400 ppm), exposure of M. beryllina embryos to ∼1,000 ppm until one week post-hatch reduced average survival and length by 74% and 18%, respectively. The egg stage was significantly more vulnerable to high CO2-induced mortality than the post-hatch larval stage. These findings challenge the belief that ocean acidification will not affect fish populations, because even small changes in early life survival can generate large fluctuations in adult-fish abundance9,10.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Doney, S. C., Fabry, V. J., Feely, R. A. & Kleypas, J. A. Ocean acidification: The other CO2 problem. Annu. Rev. Mar. Sci. 1, 169–192 (2009).
Orr, J. C. et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437, 681–686 (2005).
Kleypas, J. A. et al. Impacts of Ocean Acidification on Coral Reefs and Other Marine Calcifiers: A Guide for Future Research (Report of a workshop held 18–20 April 2005, St. Petersburg, FL, Sponsored by NSF, NOAA, and the US Geological Survey, 2006).
Fabry, V. J., Seibel, B. A., Feely, R. A. & Orr, J. C. Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J. Mar. Res. 65, 414–432 (2008).
Talmage, S. C. & Gobler, C. J. Effects of past, present, and future ocean carbon dioxide concentrations on the growth and survival of larval shellfish. Proc. Natl Acad. Sci. USA 107, 17246–17251 (2010).
Kurihara, H., Kato, S. & Ishimatsu, A. Effects of increased seawater p CO 2 on early development of the oyster Crassostrea gigas. Aquat. Biol. 1, 91–98 (2007).
Ishimatsu, A., Hayashi, M. & Kikkawa, T. Fishes in high-CO2, acidified oceans. Mar. Ecol. Prog. Ser. 373, 295–302 (2008).
Caldeira, K. & Wickett, M. E. Anthropogenic carbon and ocean pH. Nature 425, 365–365 (2003).
Sissenwine, M. P. in Exploitation of Marine Communities (ed. May, R.) 59–94 (Springer, 1984).
Trippel, E. A. & Chambers, R. C. in Early Life History and Recruitment in Fish Populations (eds Chambers, R. C. & Trippel, E. A.) xxi–xxxii (Chapman & Hall, 1997).
www.esrl.noaa.gov/gmd/ccgg/trends/.
Tripati, A. K., Roberts, C. D. & Eagle, R. A. Coupling of CO2 and ice sheet stability over major climate transitions of the last 20 million years. Science 326, 1394–1397 (2009).
Spero, H. J., Bijma, J., Lea, D. W. & Bemis, B. E. Effect of seawater carbonate concentration on foraminiferal carbon and oxygen isotopes. Nature 390, 497–500 (1997).
Riebesell, U. et al. Reduced calcification of marine plankton in response to increased atmospheric CO2 . Nature 407, 364–367 (2000).
Kurihara, H., Matsui, M., Furukawa, H., Hayashi, M. & Ishimatsu, A. Long-term effects of predicted future seawater CO2 conditions on the survival and growth of the marine shrimp Palaemon pacificus. J. Exp. Mar. Biol. Ecol. 367, 41–46 (2008).
Dupont, S., Havenhand, J., Thorndyke, W., Peck, L. & Thorndyke, M. C. Near-future level of CO2-driven ocean acidification radically affects larval survival and development in the brittlestar Ophiothrix fragilis. Mar. Ecol. Prog. Ser. 373, 285–294 (2008).
Hayashi, M., Kita, J. & Ishimatsu, A. Acid–base responses to lethal aquatic hypercapnia in three marine fishes. Mar. Biol. 144, 153–160 (2004).
Munday, P. L. et al. Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proc. Natl Acad. Sci. USA 106, 1848–1852 (2009).
Munday, P. L. et al. Replenishment of fish populations is threatened by ocean acidification. Proc. Natl Acad. Sci. USA 107, 12930–12934 (2010).
Dixson, D. L., Munday, P. L. & Jones, G. P. Ocean acidification disrupts the innate ability of fish to detect predator olfactory cues. Ecol. Lett. 13, 68–75 (2010).
Checkley, D. M. et al. Elevated CO2 enhances otolith growth in young fish. Science 324, 1683–1683 (2009).
Munday, P. L., Gagliano, M., Donelson, J. M., Dixson, D. L. & Thorrold, S. R. Ocean acidification does not affect the early life history development of a tropical marine fish. Mar. Ecol. Prog. Ser. 423, 211–221 (2011).
Riebesell, U., Fabry, V. J., Hansson, L. & Gattuso, J. P. in Guide to Best Practices for Ocean Acidification Research and Data Reporting (Publications Office of the European Union, 2010).
Anderson, J. T. A review of size dependent survival during pre-recruit stages of fishes in relation to recruitment. J. Northw. Atl. Fish. Sci. 8, 55–66 (1988).
Leggett, W. C. & Deblois, E. Recruitment in marine fishes: Is it regulated by starvation and predation in the egg and larval stages? Neth. J. Sea Res. 32, 119–134 (1994).
Mangor-Jensen, A. Water balance in developing eggs of the cod Gadus morhua L. Fish Physiol. Biochem. 3, 17–24 (1987).
Perry, S. F. & Gilmour, K. M. Acid–base balance and CO2 excretion in fish: Unanswered questions and emerging models. Respir. Physiol. Neurobiol. 154, 199–215 (2006).
Hemmer, M. J., Middaugh, D. P. & Moore, J. C. Effects of temperature and salinity on Menidia beryllina embryos exposed to terbufos. Dis. Aquat. Org. 8, 127–136 (1990).
Feely, R. A., Sabine, C. L., Hernandez-Ayon, J. M., Ianson, D. & Hales, B. Evidence for upwelling of corrosive ‘acidified’ water onto the continental shelf. Science 320, 1490–1492 (2008).
Salisbury, J., Green, M., Hunt, C. & Campbell, J. Coastal acidification by rivers: A threat to shellfish? EOS Trans. AGU 89 http://dx.doi.org/10.1029/2008EO500001 (2008).
Acknowledgements
We acknowledge the assistance of L. Merlo, R. Anderson, R. Light and Y. Tang. This work was supported by grants from the New Tamarind Foundation and National Science Foundation Biological Oceanography (no 1129622).
Author information
Authors and Affiliations
Contributions
H.B., S.C.T. and C.J.G. designed the experiments, conducted the experiments, generated the data, analysed samples, analysed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 392 kb)
Rights and permissions
About this article
Cite this article
Baumann, H., Talmage, S. & Gobler, C. Reduced early life growth and survival in a fish in direct response to increased carbon dioxide. Nature Clim Change 2, 38–41 (2012). https://doi.org/10.1038/nclimate1291
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nclimate1291
This article is cited by
-
Resiliency of black sea bass, Centropristis striata, early life stages to future high CO2 conditions
Environmental Biology of Fishes (2024)
-
Multistressor global change drivers reduce hatch and viability of Lingcod embryos, a benthic egg layer in the California Current System
Scientific Reports (2022)
-
Distribution and dynamics of particulate organic matter in Indian mangroves during dry period
Environmental Science and Pollution Research (2022)
-
The effects of CO2 level and temperature on embryos and free embryos of the Patagonian pejerrey Odontesthes hatcheri (Actinopterygii, Atherinopsidae)
Hydrobiologia (2022)
-
Swimming performance of sharks and rays under climate change
Reviews in Fish Biology and Fisheries (2022)