Abstract
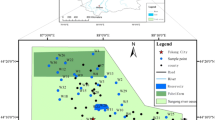
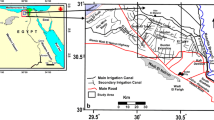
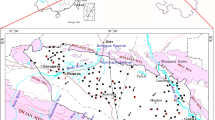
Conventional hydrochemical techniques and statistical analyses were applied to better understand the solute geochemistry and the hydrochemical process of shallow groundwater in the Qinghai Lake catchment. Shallow groundwater in the Qinghai Lake catchment is slightly alkaline, and is characterized by a high ion concentrations and low water temperature. The total dissolved solids (TDS) in most of the samples are <1,000 mg/L, i.e. fresh water and depend mainly on the concentration of SO4 2−, Cl− and Na+. Groundwater table is influenced directly by the residents’ groundwater consumption. Most of the groundwaters in the Qinghai Lake catchment belong to the Ca2+(Na+) –HCO3 − type, while the Qinghai Lake, part of the Buha (BHR) and the Lake Side (LS) samples belong to the Na+–Cl− type. The groundwater is oversaturated with respect to aragonite, calcite and dolomite, but not to magnesite and gypsum. Solutes are mainly derived from strong evaporite dissolution in Daotang, BHR and LS samples and from strong carbonate weathering in Hargai and Shaliu samples. Carbonate weathering is stronger than evaporite dissolution with weak silicate weathering in the Qinghai Lake catchment. Carbonate weathering, ion exchange reaction and precipitation are the major hydrogeochemical processes responsible for the solutes in the groundwater in the Qinghai Lake catchment. Most of the shallow groundwaters are suitable for drinking. More attention should be paid to the potential pollution of nitrate, chloride and sulfide in shallow groundwater in the future.






Similar content being viewed by others
References
Adams S, Titus R, Pietersen K, Tredoux G, Harris C (2001) Hydrochemical characteristics of aquifers near Sutherland in the Western Karoo, South Africa. J Hydrol 241:91–103
Agrawal GD, Lunkad SK, Malkhed T (1999) Diffuse agricultural nitrate pollution of groundwaters in India. Water Sci Technol 39:67–75
Ahmad T, Khanna PP, Chakrapani GJ, Balakrishnan S (1998) Geochemical characteristics of water and sediment of the Indus River, Trans-Himalaya, India: const rains on weathering and erosion. J Asian Earth Sci 16:333–346
Bethke CM, Yeakel S (2009) The Geochemist’s Workbench®, Version 8.0. Hydrogeology Program. University of Illinois, Urbana, p 84
Chang J, Wang GX (2010) Major ions chemistry of groundwater in the arid region of Zhangye Basin, northwestern China. Environ Earth Sci 61:539–547
Colman SM, Yu S, An ZS, Shen J, Henderson ACG (2007) Late Cenozoic climate changes in China’s western interior: a review of research on Lake Qinghai and comparison with other records. Quat Sci Rev 26:2281–2300
Deutsch WJ (1997) Groundwater geochemistry: fundamentals and application to contamination. CRC, Boca Raton
Edmunds WM, Ma JZ, Aeschbach-Hertig W, Kipferd R, Darbyshire DPF (2006) Groundwater recharge history and hydrogeochemical evolution in the Minqin Basin, North West China. Appl Geochem 21:2148–2170
Fisher RS, Mulican WF III (1997) Hydrochemical evolution of sodium-sulphate and sodium-chloride groundwater beneath the Northern Chihuahuan desert Trans-Pecos, Texas, USA. Hydrogeol J 10:455–474
Gaillardet J, Dupré B, Louvat P, Allegre CJ (1999) Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem Geol 159:3–30
Galy A, France-Lanord C (1999) Weathering processes in the Ganges-Brahmaputra basin and the riverine alkalinity budget. Chem Geol 159:31–60
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Sci J 170:795–840
Guo HM, Wang YX (2005) Geochemical characteristics of shallow groundwater in Datong basin, northwestern China. J Geochem Explor 87:109–120
Han GL, Liu CQ (2004) Water geochemistry controlled by carbonate dissolution: a study of the river waters draining karst-dominated terrain, Guizhou Province, China. Chem Geol 204:1–21
Huh Y, Tsoi MY, Zaitsev A, Edmond JM (1998) The fluvial geochemistry of the rivers of Eastern Siberia: I. Tributaries of the Lena River draining the sedimentary platform of the Siberian Craton. Geochim Cosmochim Acta 62:1657–1676
Jankowski J, Acworth RI (1997) Impact of debris-flow deposits on hydrogeochemical processes and the development of dryland salinity in the Yass River catchment, New South Wales, Australia. Hydrogeol J 5:71–88
Jin ZD, Yu JM, Wang SM, Zhang F, Shi YW, You CF (2009a) Constraints on water chemistry by chemical weathering in the Qinghai Lake catchment, northeastern Tibetan Plateau (China): clues from Sr and its isotopic geochemistry. Hydrogeol J 17:2037–2048
Jin ZD, You CF, Yu JM (2009b) Toward a geochemical mass balance of major elements in Lake Qinghai, NE Tibetan Plateau: a significant role of atmospheric deposition. Appl Geochem 24:1901–1907
Jin ZD, Wang SM, Zhang F, Shi YW (2010a) Weathering, Sr fluxes, and controls on water chemistry in the Qinghai Lake catchment, NE Tibetan Plateau. Earth Surf Proc Land 35:1057–1070
Jin ZD, You CF, Wang Y, Shi YW (2010b) Hydrological and solute budgets of Lake Qinghai, the largest lake on the Tibetan Plateau. Quat Int 218:151–156
Jin ZD, You CF, Yu JM, Wu LL, Zhang F, Liu HC (2011) Seasonal contributions of catchment weathering and eolian dust to river water chemistry, northeastern Tibetan Plateau: chemical and Sr isotopic constraints. J Geophys Res 116:F04006. doi:10.1029/2011JF002002
Li CL, Kang SC, Zhang QG, Kaspari S (2007a) Major ionic composition of precipitation in the Nam Co region, central Tibetan Plateau. Atmos Res 85:351–360
Li XY, Xu H, Sun YL, Zhang DS, Yang ZP (2007b) Lake-level change and water balance analysis at Lake Qinghai, west China during recent decades. Water Resour Manag 21:1505–1516
Liu WG, Li XZ, Zhang L, An ZS, Xu LM (2009) Evaluation of oxygen isotopes in carbonate as an indicator of lake evolution in arid areas: the modern Qinghai Lake, Qinghai-Tibet Plateau. Chem Geol 268:126–136
Ma JZ, Wang XS, Edmunds WM (2005) The characteristics of ground-water resources and their changes under the impacts of human activity in the arid Northwest China—a case study of the Shiyang River Basin. J Arid Environ 61:277–295
Meybeck M (1987) Global chemical weathering of surficial rocks estimated from river dissolved loads. Am J Sci 287:401–428
Ministry of Health (2006) Standards for drinking water quality. GB5749-2006. Ministry of Health of the People’s Republic of China, Beijing
Négrel P, Allègre CJ, Dupré B, Lewin E (1993) Erosion sources determined by inversion of major and trace element ratios and strontium isotopic ratios in fiver water: The Congo Basin case. Earth Planet Sci Lett 120:59–76
Qiu J (2010) China faces up to groundwater crisis. Nature 466:308
Oren O, Yechieli Y, Böhlke JK, Dody A (2004) Contamination of groundwater under cultivated fields in an arid environment, central Arava Valley, Israel. J Hydrol 290:312–328
Sami K (1992) Recharge mechanisms and geochemical processes in a semi-arid sedimentary basin, Eastern Cape, South Africa. J Hydrol 139:27–48
Sarin MM, Krishnaswami S (1984) Major ion chemistry of the Ganga-Brahamputra river systems, India. Nature 312:538–541
Shanyengana ES, Seely MK, Sanderson RD (2004) Major-ion chemistry and ground-water salinization in ephemeral floodplains in some arid regions of Namibia. J Arid Environ 57:71–83
Sun Y, Li X, Xu H (2007) Daily precipitation and temperature variations in the Qinghai Lake watershed in recent 40 years. Arid Meteorol 25:7–13
Tizro AT, Voudouris KS (2008) Groundwater quality in the semi-arid region of the Chahardouly basin, West Iran. Hydrol Process 22:3066–3078
Wen X, Wu Y, Su J, Zhang Y, Liu F (2005) Hydrochemical characteristics and salinity of groundwater in the Ejina basin, northwestern China. Environ Geol 48:665–675
Wu LL, Huh Y, Qin JH, Du G, Lee SD (2005) Chemical weathering in the Upper Huang He (Yellow River) draining the eastern Qinghai-Tibet Plateau. Geochim Cosmochim Acta 69:5279–5294
Xu H, Hou ZH, An ZS, Liu XY, Dong JB (2010a) Major ion chemistry of waters in the Qinghai Lake catchments, NE Qinghai-Tibet plateau, China. Quat Int 212:35–43
Xu H, Liu B, Wu F (2010b) Spatial and temporal variations of Rb/Sr ratios of the bulk surface sediments in Lake Qinghai. Geochem Trans 11:3–8
Yu JQ, Kelts KR (2002) Abrupt changes in climate conditions across the lateglacial/Holocene transition on the N.E. Tibet-Qinghai Plateau: evidence from Lake Qinghai. J Paleolimnol 28:195–206
Zhang F, Jin ZD, Hu G, Li FC, Shi YW (2009) Seasonally chemical weathering and CO2 consumption flux of Lake Qinghai river system in the northeastern Tibetan Plateau. Environ Earth Sci 59:297–313
Zhang J, Takahashi K, Wushiki H, Wushiki H, Yabuki S, Xiong JM, Masuda A (1995) Water geochemistry of the rivers around the Taklimakan Desert (NW China): crustal weathering and evaporation processes in arid land. Chem Geol 119:225–2371
Zhu BQ, Yang XP (2007) The ion chemistry of surface and ground waters in the Taklimakan Desert of Tarim Basin, western China. Chin Sci Bull 52:2123–2129
Acknowledgments
This work was financially supported by National Science Foundation of China through grants 40873082 and 41003012, National Basic Research Program of China (2010CB833404) and West Light Foundation of Chinese Academy of Sciences. We especially thank Associate Professor Yuxin Zhu in Nanjing Institute of Geography & Limnology, Chinese Academy of Sciences, and Miss Ting Zhang in Institute of Earth Environment, Chinese Academy of Sciences, for their kind helps and suggestions to sample analyses and laboratory work. Thanks are extended to Mr. Yuewei Shi at the Buha River Hydrological Station for his assistance with sample collection.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiao, J., Jin, Z., Zhang, F. et al. Major ion geochemistry of shallow groundwater in the Qinghai Lake catchment, NE Qinghai-Tibet Plateau. Environ Earth Sci 67, 1331–1344 (2012). https://doi.org/10.1007/s12665-012-1576-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-012-1576-4