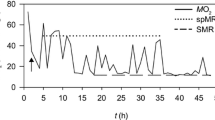
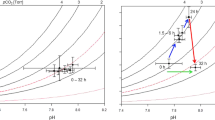
Abstract
Acidification of ocean surface waters by anthropogenic carbon dioxide (CO2) emissions is a currently developing scenario that warrants a broadening of research foci in the study of acid–base physiology. Recent studies working with environmentally relevant CO2 levels, indicate that some echinoderms and molluscs reduce metabolic rates, soft tissue growth and calcification during hypercapnic exposure. In contrast to all prior invertebrate species studied so far, growth trials with the cuttlefish Sepia officinalis found no indication of reduced growth or calcification performance during long-term exposure to 0.6 kPa CO2. It is hypothesized that the differing sensitivities to elevated seawater pCO2 could be explained by taxa specific differences in acid–base regulatory capacity. In this study, we examined the acid–base regulatory ability of S. officinalis in vivo, using a specially modified cannulation technique as well as 31P NMR spectroscopy. During acute exposure to 0.6 kPa CO2, S. officinalis rapidly increased its blood [HCO3 −] to 10.4 mM through active ion-transport processes, and partially compensated the hypercapnia induced respiratory acidosis. A minor decrease in intracellular pH (pHi) and stable intracellular phosphagen levels indicated efficient pHi regulation. We conclude that S. officinalis is not only an efficient acid–base regulator, but is also able to do so without disturbing metabolic equilibria in characteristic tissues or compromising aerobic capacities. The cuttlefish did not exhibit acute intolerance to hypercapnia that has been hypothesized for more active cephalopod species (squid). Even though blood pH (pHe) remained 0.18 pH units below control values, arterial O2 saturation was not compromised in S. officinalis because of the comparatively lower pH sensitivity of oxygen binding to its blood pigment. This raises questions concerning the potentially broad range of sensitivity to changes in acid–base status amongst invertebrates, as well as to the underlying mechanistic origins. Further studies are needed to better characterize the connection between acid–base status and animal fitness in various marine species.






Similar content being viewed by others
References
Bock C, Sartoris FJ, Wittig RM, Pörtner HO (2001) Temperature-dependent pH regulation in stenothermal antaractic and eurythermal temperate eelpout (Zoarcidae): an in vivo NMR study. Polar Biol 24:869–874
Bock C, Sartoris FJ, Pörtner HO (2002) In vivo MR spectroscopy and MR imaging on non-anaesthetized marine fish: techniques and first results. Magn Reson Imaging 20:165–172
Bohr C, Hasselbalchk A, Krogh A (1904) Über einen in biologischer Beziehung wichtigen Einfluss, den die Kohlensäurespannung des Blutes auf dessen Sauerstof f bindung übt. Skand Arch Physiol 16:402–412
Bone Q, Brown ER, Travers G (1994) On the respiratory flow in the cuttlefish Sepia officinalis. J Exp Biol 194:153–165
Boron WF (2004) Regulation of intracellular pH. Adv Physiol Educ 28:160–179
Boutilier RG, Iwama GK, Heming TA, Randall DJ (1985) The apparent pk of carbonic-acid in rainbow-trout blood-plasma between 5°C and 15°C. Resp Physiol 61:237–254
Bridges CR (1994) Bohr and Root effects in cephalopod haemocyanins-paradox or pressure in Sepia officinalis? In: Pörtner HO, O’Dor RK, MacMillan D (eds) Physiology of cephalopod molluscs. Gordon and Breach, Basel, pp 121–130
Cameron JN (1986) Acid–base equilibria in invertebrates. In: Heisler N (ed) Acid–base regulation in animals. Elsevier Biomedical Press, Amsterdam, pp 357–394
Cameron JN, Iwama GK (1987) Compensation of progressive hypercapnia in channel catfish and blue crabs. J Exp Biol 133:183–197
Claiborne JB (1998) Acid–base regulation. In: Evans DH (ed) The physiology of fishes. CRC Press, Boca Raton, FL, pp 179–200
Claiborne JB, Evens DE (1992) Acid–base balance and ion transfers in the spiny dogfish (Squalus acanthias) during hypercapnia: a role for ammonia excretion. J Exp Zool 261:9–17
Deigweiher K, Koschnick N, Pörtner HO, Lucassen M (2008) Acclimation of ion regulatory capacities in gills of marine fish under environmental hypercapnia. Am J Regul Integr Comp Physiol 295:1660–1670
Dickson AG, Millero FJ (1987) A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep Sea Res 34:1733–1743
Doumen EJ, Ellington WR (1992) Intracellular free magnesium in the muscle of an osmoconforming marine invertebrate: measurement and effect of metabolic and acid–base perturbations. J Exp Biol 261:394–405
Dubyak GR (2004) Ion homeostasis, channels, and transporters: an update on cellular mechanisms. Adv Physiol Educ 28:143–154
Dwyer JJ, Burnett LE (1996) Acid–base status of the oyster Crassostrea virginica in response to air exposure and to infections by Perkinsus marinus. Biol Bull 190:139–147
Evans DH, Piermarini PM, Choe KP (2005) The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid–base regulation, and excretion of nitrogenous waste. Physiol Rev 85:97–177
Fabry VJ, Seibel BA, Feely RA, Orr JC (2008) Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J Mar Sci 65:414–432
Fivelstad S, Olsen AB, Asgard T, Baeverfjord G, Rasmussen T, Vindheim T, Stefansson S (2003) Long-term sublethal effects of carbon dioxide on Atlantic salmon smolts (Salmo salar L.): ion regulation, haematology, element composition, nephrocalcinosis and growth parameters. Aquaculture 215:301–319
Foss A, Rosnes BA, Oiestad V (2003) Graded environmental hypercapnia in juvenile spotted wolffish (Anarhichas minor Olafsen): effects on growth, food conversion efficiency and nephrocalcinosis. Aquaculture 220:607–617
Gutowska MA, Pörtner HO, Melzner F (2008) Growth and calcification in the cephalopod Sepia officinalis under elevated seawater pCO2. Mar Ecol Prog Ser 373:303–309
Heisler N (1986) Acid–base regulation in fishes. In: Heisler N (ed) Acid–base regulation in animals. Elsevier Biomedical Press, Amsterdam, pp 309–356
Heisler N (1989) Interaction between gas exchange, metabolism, and ion transport in animals: an overview. Can J Zool 67:293–2935
Henry RP, Cameron JN (1983) The role of carbonic-anhydrase in respiration, ion regulation and acid–base balance in the aquatic crab Callinectes sapidus and the terrestrial crab Gecarcinus lateralis. J Exp Biol 103:205–223
Intergovernmental Panel on Climate Change (IPPC) (2007) Climate Change 2007 Synthesis Report. Cambridge University Press, New York
Johansen K, Brix O, Lykkeboe G (1982) Blood-gas transport in the cephalopod, Sepia officinalis. J Exp Biol 99:331–338
Kammermeier H, Schmidt P, Jüngling E (1982) Free energy change of ATP-hydrolysis: a causal factor of early hypoxic failure of the myocardium? J Mol Cell Cardio 14:267–277
Kost GJ (1990) pH standardization for phosphorus-31 magnetic resonance heart spectroscopy at different temperatures. Magn Res Med 14:496–506
Larsen BK, Pörtner HO, Jensen FB (1997) Extra- and intracellular acid–base balance and ionic regulation in cod (Gadus morhua) during combined and isolated exposures to hypercapnia and copper. Mar Biol 128:337–346
Lenfant C, Aucutt C (1966) Measurement of blood gases by gas chromatography. Resp Physiol 1:398–407
Lewis E, Wallace DWR (1998) Program developed for CO2 system calculations. ORNL/CDIAC-105, Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, Oak Ridge, TN. http://cdiac.esd.ornl.gov/ocoeans/co2rprt.html
Lindinger MI, Lauren DJ, McDonald DG (1984) Acid–base balance in the sea mussel, Mytilus edulis.3. Effects of environmental hypercapnia on intracellular and extracellular acid–base balance. Mar Biol Lett 5:371–381
Mehrbach C, Culberso CH, Hawley JE, Pytkowic RM (1973) Measurement of apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol Oceanogr 18:897–907
Melzner F (2005) Systemic investigations on the physiology of temperature tolerance in the common cuttlefish Sepia officinalis. Ph.D. thesis, University of Bremen, Germany
Melzner F, Bock C, Pörtner HO (2006a) Critical temperatures in the cephalopod Sepia officinalis investigated using in vivo (31)P-NMR spectroscopy. J Exp Biol 209:891–906
Melzner F, Bock C, Pörtner HO (2006b) Temperature-dependent oxygen extraction from the ventilatory current and the costs of ventilation in the cephalopod Sepia officinalis. J Comp Physiol B 176:607–621
Melzner F, Bock C, Pörtner HO (2007a) Coordination between ventilatory pressure oscillations and venous return in the cephalopod Sepia officinalis under control conditions, spontaneous exercise and recovery. J Comp Physiol B 177:1–17
Melzner F, Mark FC, Pörtner HO (2007b) Role of blood-oxygen transport in thermal tolerance of the cuttlefish, Sepia officinalis. Integr Comp Biol 47:645–655
Melzner F, Gutowska MA, Langenbuch M, Dupont S, Lucassen M, Thorndyke M, Bleich M, Pörtner HO (2009) Physiological basis for high CO2 tolerance in marine ectothermic animals: pre-adaptation through lifestyle and ontogeny? Biogeosciences (in press)
Messenger JB, Nixon M, Ryan KP (1985) Magnesium chloride as an anaesthetic for cephalopods. Comp Biochem Phys C 82:203–205
Michaelidis B, Ouzounis C, Paleras A, Pörtner HO (2005) Effects of long-term moderate hypercapnia on acid–base balance and growth rate in marine mussels Mytilus galloprovincialis. Mar Ecol Prog Ser 293:109–118
Michaelidis B, Spring A, Pörtner OH (2007) Effects of long-term acclimation at hypercapnia on acid-base status and metabolism in tissues of Sparus aurata. Mar Biol 150(6):1417–1429
Miles H, Widdicombe S, Spicer JI, Hall-Spencer J (2007) Effects of anthropogenic seawater acidification on acid–base balance in the sea urchin Psammechinus miliaris. Mar Pollut Bull 54:89–96
O’Dor RK, Webber DM (1986) The constraints on cephalopods: why squid aren’t fish. Can J Zool 64:1591–1605
Pane EF, Barry JP (2007) Extracellular acid–base regulation during short-term hypercapnia is effective in a shallow-water crab, but ineffective in a deep-sea crab. Mar Ecol Prog Ser 334:1–9
Pörtner HO (1990) An analysis of the effects of pH on oxygen binding by squid (Illex illecebrosus, Loligo pealei) hemocyanin. J Exp Biol 150:407–424
Pörtner HO (1994) Coordination of metabolism, acid–base regulation and haemocyanin function in cephalopods. Mar Freshw Behav Phy 25:131–148
Pörtner HO (2008) Ecosystem effects of ocean acidification in times of ocean warming: a physiologist’s view. Mar Ecol Prog Ser 373:203–217
Pörtner HO, Boutilier RG, Tang Y, Toews DP (1990) Determination of intracellular pH and pCO2 after metabolic inhibition by fluoride and nitrilotriacetic acid. Resp Physiol 81:255–274
Pörtner HO, Webber DM, Boutilier RG, Odor RK (1991) Acid–base regulation in exercising squid (Illex illecebrosus, Loligo pealei). Am J Physiol 261:239–246
Pörtner HO, Reipschlager A, Heisler N (1998) Acid–base regulation, metabolism and energetics in Sipunculus nudus as a function of ambient carbon dioxide level. J Exp Biol 201:43–55
Pörtner HO, Bock C, Reipschlager A (2000) Modulation of the cost of pHi regulation during metabolic depression: a (31)P-NMR study in invertebrate (Sipunculus nudus) isolated muscle. J Exp Biol 203:2417–2428
Pörtner HO, Langenbuch M, Reipschlager A (2004) Biological impact of elevated ocean CO2 concentrations: lessons from animal physiology and earth history. J Oceanogr 60:705–718
Redfield AC, Goodkind R (1929) The significance of the Bohr effect in the respiration and asphyxiation of the squid, Loligo pealei. J Exp Biol 6:340–349
Reeves RB (1972) An imidazole alphastat hypothesis for vertebrate acid–base regulation: tissue carbon dioxide content and body temperature in bullfrogs. Resp Physiol 14:219–236
Reipschlager A, Pörtner HO (1996) Metabolic depression during environmental stress: the role of extracellular versus intracellular pH in Sipunculus nudus. J Exp Biol 199:1801–1807
Rosa R, Seibel BA (2008) Synergistic effects of climate-related variables suggest future physiological impairment in a top oceanic predator. Proc Natl Acad Sci USA 105:20776–20780
Shirayama Y, Thornton H (2005) Effect of increased atmospheric CO2 on shallow water marine benthos. J Geophys Res Oceans 110
Storey KB, Storey JM (1979) Octopine metabolism in the cuttlefish, Sepia officinalis - Octopine production by muscle and its role as an aerobic substrate for non-muscular tissues. J Comp Physiol 131:311–319
Toews DP, Holeton GF, Heisler N (1983) Regulation of acid–base status during environmental hypercapnia in the marine teleost fish Conger conger. J Exp Biol 107:9–20
Tompsett DH (1939) Sepia. University Press of Liverpool, Liverpool
Truchot JP (1976) Carbon dioxide combining properties of blood of shore crab Carcinus maenas-L—carbon dioxide solubility coefficient and carbonic acid dissociation constants. J Exp Biol 64:45–57
Truchot JP (1984) Water carbonate alkalinity as a determinant of hemolymph acid–base balance in the shore crab, Carcinus maenas—a study at two different ambient pCO2 and pO2 levels. J Comp Physiol 154:601–606
Wells MJ, Wells J (1991) Is Sepia really an octopus? In: Boucaud-Camou E (ed) La Seiche, 1st international symposium on the cuttlefish Sepia. Centre de publications, Universite de Caen, pp 7–92
Whiteley NM, Scott JL, Breeze SJ, McCann L (2001) Effects of water salinity on acid–base balance in decapod crustaceans. J Exp Biol 204:1003–1011
Zielinski S, Sartoris FJ, Pörtner HO (2001) Temperature effects on hemocyanin oxygen binding in an Antarctic cephalopod. Biol Bull 200:67–76
Acknowledgments
This study was supported by a Fulbright Fellowship (MAG), the AWI ‘MARCOPOLI’ Program (MAG, FM, ML, FJS, CB, HOP), and the DFG Excellence Cluster ‘Future Ocean’ (FM, ML). We extend our thanks to R. Wittig for NMR data analysis. This work is a contribution to the German Ministry of Education and Research (BMBF) funded project “Biological Impacts of Ocean ACIDification” (BIOACID) Subproject 3.1.3 and the “European Project on Ocean Acidification” (EPOCA) which received funding from the European Community’s Seventh Framework Programme (FP7/2007-2013) under grant agreement n° 211384.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gutowska, M.A., Melzner, F., Langenbuch, M. et al. Acid–base regulatory ability of the cephalopod (Sepia officinalis) in response to environmental hypercapnia. J Comp Physiol B 180, 323–335 (2010). https://doi.org/10.1007/s00360-009-0412-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-009-0412-y