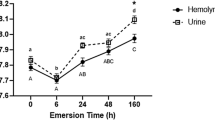
Abstract
Brachyuran and anomuran decapod crabs do not occur in the extremely cold waters of the Antarctic continental shelf whereas caridean and other shrimp-like decapods, amphipods and isopods are highly abundant. Differing capacities for extracellular ion regulation, especially concerning magnesium, have been hypothesised to determine cold tolerance and by that the biogeography of Antarctic crustaceans. Magnesium is known to have a paralysing effect, which is even more distinct in the cold. As only few or no data exist on haemolymph ionic composition of Sub-Antarctic and Antarctic crustaceans, haemolymph samples of 12 species from these regions were analysed for the concentrations of major inorganic ions (Na+, K+, Ca2+, Mg2+, Cl−, SO4 2−) by ion chromatography. Cation relationships guaranteed neuromuscular excitability in all species. Sulphate and potassium correlated positively with magnesium concentration. The Antarctic caridean decapod as well as the amphipods maintained low (6–20% of ambient sea water magnesium concentration), Sub-Antarctic brachyuran and anomuran crabs as well as the Antarctic isopods high (54–96% of ambient sea water magnesium concentration) haemolymph magnesium levels. In conclusion, magnesium regulation may explain the biogeography of decapods, but not that of the peracarids.



Similar content being viewed by others
References
Arntz WE, Gutt J, Klages M (1997) Antarctic marine biodiversity: an overview. In: Battaglia B, Valencia J, Walton DWH (eds) Antarctic communities: species, structure and survival. Cambridge University Press, Cambridge, pp 3–14
Aronson RB, Thatje S, Clarke A, Peck LS, Blake DB, Wilga CD, Seibel BA (2007) Climate change and invasibility of the Antarctic benthos. Annu Rev Ecol Syst 38:129–154
Atkinson MJ, Bingman C (1997) Elemental composition of commercial seasalts. J Aquacult Aquat Sci 8:39–43
Barnes D, Peck LS (2008) Vulnerability of Antarctic shelf biodiversity to predicted regional warming. Clim Res 37:149–163
Bock C, Frederich M, Wittig R-M, Pörtner HO (2001) Simultaneous observations of haemolymph flow and ventilation in marine spider crabs at different temperatures: a flow weighted MRI study. Magn Reson Imaging 19:1113–1124
Brandt A (1999) On the origin and evolution of Antarctic Peracarida. Sci Mar 63:261–274
Brey T, Dahm C, Gorny M, Klages M, Stiller M, Arntz WE (1996) Do Antarctic benthic invertebrates show an extended level of eurybathy? Antarct Sci 8:3–6
Burton RF (1995) Cation balance in crustacean haemolymph: relationship to cell membrane potentials and membrane surface charge. Comp Biochem Physiol A 111:125–131
Clarke A (1988) Seasonality in the Antarctic marine environment. Comp Biochem Physiol B 90:461–473
Clarke A, Barnes DKA, Hodgson DA (2005) How isolated is Antarctica? Trends Ecol Evol 20:1–3
Clarke A, Griffiths HJ, Barnes DKA, Meredith MP, Grant SM (2009) Spatial variation in seabed temperatures in the Southern Ocean: implications for benthic ecology and biogeography. J Geophys Res 114:G03003. doi:10.1029/2008JG000886
Copeland DE, Fitzjarrell AT (1968) The salt absorbing cells in the gills of the blue crab (Callinectes sapidus Rathbun) with notes on modified mitochondria. Z Zellforsch 92:1–22
Dall W (1974) Indices of nutritional state in the western rock lobster, Panulirus longipes (Milne Edwards). I. Blood and tissue constituents and water content. J Exp Mar Biol Ecol 16:167–180
Dunn TW, Mercier AJ (2003) Synaptic modulation by a neuropeptide depends on temperature and extracellular calcium. J Neurophysiol 89:1807–1814
Eckert R, Randall D, Burggren WW, French K (2000) Tierphysiologie. Thieme, Stuttgart
Feldmann RM, Zinsmeister WJ (1984a) First occurrence of fossil decapod crustaceans (Callianassidae) from the McMurdo Sound region, Antarctica. J Paleontol 58:1041–1045
Feldmann RM, Zinsmeister WJ (1984b) New fossil crabs (Decapoda: Brachyura) from the La Meseta Formation (Eocene) of Antarctica: paleogeographic and biogeographic implications. J Paleontol 58:1046–1061
Frederich M (1999) Ecophysiological limits to the geographical distribution of reptant decapod crustaceans in the Antarctic. Rep Polar Res 335
Frederich M, Pörtner HO (2000) Oxygen limitation of thermal tolerance defined by cardiac and ventilatory performance in the spider crab, Maja squinado. Am J Physiol Regul Integr Comp Physiol R 279:1531–1538
Frederich M, DeWachter B, Sartoris FJ, Pörtner HO (2000a) Cold tolerance and the regulation of cardiac performance and hemolymph distribution in Maja squinado (Crustacea: Decapoda). Physiol Biochem Zool 73:406–415
Frederich M, Sartoris FJ, Arntz WE, Pörtner HO (2000b) Haemolymph Mg2+ regulation in decapod crustaceans: physiological correlates and ecological consequences in polar areas. J Exp Biol 203:1383–1393
Freire CA, Onken H, McNamara JC (2008) A structure-function analysis of ion transport in crustacean gills and excretory organs. Comp Biochem Physiol A 151:272–304
F.-Tsukamoto Y, Kuwasawa K, Takeuchi S, Mano M (2000) Physiological saline suitable for the marine isopod crustacean Bathynomus doederleini. Zool Sci 17:425–430
García Raso JE, Manjón-Cabeza ME, Ramos A, Olaso I (2005) New record of Lithodidae (Crustacea, Decapoda, Anomura) from the Antarctic (Bellingshausen Sea). Polar Biol 28:642–646
Gerencser GA, Ahearn GA, Zhang J, Cattey MA (2001) Sulfate transport mechanisms in epithelial systems. J Exp Zool 289:245–253
Gorny M (1999) On the biogeography and ecology of the Southern Ocean decapod fauna. Sci Mar 63:367–382
Gutt J, Gorny M, Arntz WE (1991) Spatial distribution of Antarctic shrimps (Crustacea: Decapoda) by underwater photography. Antarct Sci 3:363–369
Gutt J, Sirenko BI, Smirnov IS, Arntz WE (2004) How many macrozoobenthic species might inhabit the Antarctic shelf? Antarct Sci 16:11–16
Hall S, Thatje S (2009) Global bottlenecks in the distribution of marine Crustacea: temperature constraints in the family Lithodidae. J Biogeogr. doi:10.1111/j.1365-2699.2009.02153.x
Heilmayer O, Thatje S, McClelland C, Conlan K, Brey T (2008) Changes in biomass and elemental composition during early ontogeny of the Antarctic isopod crustacean Ceratoserolis trilobitoides. Polar Biol 31:1325–1331
Held C (2000) Phylogeny and biogeography of serolid isopods (Crustacea, Isopoda, Serolidae) and the use of ribosomal expansion segments in molecular systematics. Mol Phylogen Evol 15:165–178
Iseri LT, French JH (1984) Magnesium: nature’s physiologic calcium blocker. Am Heart J 108:188–193
Janssen HH, Hoese B (1993) Marsupium morphology and brooding biology of the Antarctic giant isopod Glyptonotus antarcticus Eights 1853 (Crustacea, Isopoda, Chaetiliidae). Polar Biol 13:145–149
Katz B (1936) Neuro-muscular transmission in crabs. J Physiol 87:199–221
Khodabandeh S, Charmantier G, Charmantier-Daures M (2005) Ultrastructural Studies and Na+, K+-ATPase immunolocalization in the antennal urinary glands of the lobster Homarus gammarus (Crustacea, Decapoda). J Histochem Cytochem 53:1203–1214
Kiko R, Werner I, Wittmann A (2009) Osmotic and ionic regulation in response to salinity variations and cold resistance in the Arctic under-ice amphipod Apherusa glacialis. Polar Biol 32:393–398
Lawver LA, Gahagan LM (2003) Evolution of Cenozoic seaways in the circum-Antarctic region. Palaeogeogr Palaeoclimatol Palaeoecol 198:11–38
Lee C, Zhang X, Kwan WF (1996) Electromyographic and mechanomyographic characteristics of neuromuscular block by magnesium sulphate in the pig. Br J Anaesth 76:278–283
Locarnini RA, Mishonov AV, Antonov JI, Boyer TP, Garcia HE (2006) Temperature. In: Levitus S (ed) World Ocean Atlas 2005, vol 1. U.S. Government Printing Office, Washington D.C.
Lucu C, Towle DW (2003) Na++K+-ATPase in gills of aquatic crustacea. Comp Biochem Physiol A 135:195–214
Luxmoore RA (1982) The reproductive biology of some serolid isopods from the Antarctic. Polar Biol 1:3–11
Mackay WC, Prosser CL (1970) Ionic and osmotic regulation in the king crab and two other North Pacific crustaceans. Comp Biochem Physiol 34:273–280
Mantel LH, Farmer LL (1983) Osmotic and ionic regulation. In: Bliss DE (ed) The biology of Crustacea, vol 5. Academic Press, New York, pp 53–161
McAllen R, Taylor A, Freel J (2005) Seasonal variation in the ionic and protein content of haemolymph from seven deep-sea decapod genera from the Northeast Atlantic Ocean. Deep Sea Res (1 Oceanogr Res Pap) 52:2017–2028
Mileikovsky SA (1971) Types of larval development in marine bottom invertebrates, their distribution and ecological significance: a re-evaluation. Mar Biol 10:193–213
Morritt D, Spicer JI (1993) A brief re-examination of the function and regulation of extracellular magnesium and its relationship to activity in crustacean arthropods. Comp Biochem Physiol A 106:19–23
Neufeld GJ, Holliday CW, Pritchard JB (1980) Salinity adaptation of gill Na, K-ATPase in the blue crab, Callinectes sapidus. J Exp Zool 211:215–224
Normant M, Kubicka M, Lapucki T, Czarnowski W, Michalowska M (2005) Osmotic and ionic haemolymph concentrations in the Baltic Sea amphipod Gammarus oceanicus in relation to water salinity. Comp Biochem Physiol A 141:94–99
Orsi AH, Whitworth T, Nowlin WD (1995) On the meridional extent and fronts of the Antarctic Circumpolar Current. Deep-Sea Res (1 Oceanogr Res Pap) 42:641–673
Pantin CFA (1948) Notes on microscopocal techniques for zoologists. Cambridge University Press, Cambridge
Parnas H, Parnas I, Ravin R, Yudelevitch B (1994) Glutamate and N-methyl-d-aspartate affect release from crayfish axon terminals in a voltage-dependent manner. Proc Natl Acad Sci USA 91:11586–11590
Parry G (1953) Osmotic and ionic regulation in the isopod crustacean Ligia oceanica. J Exp Biol 30:567–574
Pörtner HO (2002) Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp Biochem Physiol A 132:739–761
Poulin E, Palma AT, Feral JP (2002) Evolutionary versus ecological success in Antarctic benthic invertebrates. Trends Ecol Evol 17:218–222
Richmond J, Sher E, Keller R, Haylett B, Reichwein B, Cooke I (1995) Regulation of calcium currents and secretion by magnesium in crustacean peptidergic neurons. Invertebr Neurosci 1:215–221
Robertson JD (1953) Further studies on ionic regulation in marine invertebrates. J Exp Biol 30:277–296
Robertson JD (1960) Ionic regulation in the crab Carcinus maenas (L.) in relation to the moulting cycle. Comp Biochem Physiol 1:183–212
Ruppert EE, Barnes RD (1994) Invertebrate zoology, 6th edn. Saunders College Publishing, Orlando
Sartoris FJ, Pörtner HO (1997a) Increased concentrations of haemolymph Mg2+ protect intracellular pH and ATP levels during temperature stress and anoxia in the common shrimp Crangon crangon. J Exp Biol 200:785–792
Sartoris FJ, Pörtner HO (1997b) Temperature dependence of ionic and acid-base regulation in boreal and arctic Crangon crangon and Pandalus borealis. J Exp Mar Biol Ecol 211:69–83
Sartoris FJ, Frederich M, Pörtner HO (1997) Does the capability to regulate magnesium determine the composition of the Antarctic crustacean fauna? Verh Dtsch Zool Ges 90:144
Spicer JI, Morritt D, Taylor AC (1994) Effect of low temperature on oxygen uptake and haemolymph ions in the sandhopper Talitrus saltator (Crustacea: Amphipoda). J Mar Biol Assoc UK 74:313–321
Taylor AC, Moore PG (1995) The burrows and physiological adaptations to a burrowing lifestyle of Natatolana borealis (Isopoda: Cirolanidae). Mar Biol 123:805
Tentori E, Lockwood APM (1990) Haemolymph magnesium levels in some oceanic Crustacea. Comp Biochem Physiol A 95:545–548
Thatje S, Fuentes V (2003) First record of anomuran and brachyuran larvae (Crustacea: Decapoda) from Antarctic waters. Polar Biol 26:279–282
Thatje S, Schnack-Schiel S, Arntz WE (2003) Developmental trade-offs in Subantarctic meroplankton communities and the enigma of low decapod diversity in high southern latitudes. Mar Ecol Prog Ser 260:195–207
Thatje S, Anger K, Calcagno GA, Lovrich GA, Pörtner HO, Arntz WE (2005) Challenging the cold: crabs reconquer the Antarctic. Ecology 86:619–625
Thatje S, Hall S, Hauton C, Held C, Tyler P (2008) Encounter of lithodid crab Paralomis birsteini on the continental slope off Antarctica, sampled by ROV. Polar Biol 31:1143–1148
Walters NJ, Uglow RF (1981) Haemolymph magnesium and relative heart activity of some species of marine decapod crustaceans. J Exp Mar Biol Ecol 55:255–265
Waterman TH (1941) A comparative study of the effects of ions on whole nerve and isolated single nerve fiber preparations of crustacean neuromuscular systems. J Cell Comp Physiol 18:109–126
Watt AJS, Whiteley NM, Taylor EW (1999) An in situ study of respiratory variables in three British sublittoral crabs with different routine rates of activity. J Exp Mar Biol Ecol 239:1–21
Young JS, Peck LS, Matheson T (2006) The effects of temperature on walking and righting in temperate and Antarctic crustaceans. Polar Biol 29:978–987
Zanders IP (1980) The control of magnesium and sulphate excretion in Carcinus maenas (L.). Comp Biochem Physiol A 66:69–76
Ziegler A, Grospietsch T, Carefoot TH, Danko JP, Zimmer M, Zerbst-Boroffka I, Pennings SC (2000) Hemolymph ion composition and volume changes in the supralittoral isopod Ligia pallasii Brandt, during molt. J Comp Physiol B 170:329–336
Acknowledgments
We would like to thank Timo Hirse and Florian Leese for sample collection. We greatly appreciate the help of the crew of RV Polarstern during the transport of live animals. The experiments comply with the current laws of the country in which they were performed. This study was supported by Deutsche Forschungsgemeinschaft grants no. SA 1713 and He 3391/3 and by National Science Foundation grant no. OPP V01-32032. This is publication 25 of the ICEFISH cruise 2004.
Conflict of interest statement
The authors declare that they have no conflict of interest
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wittmann, A.C., Held, C., Pörtner, H.O. et al. Ion regulatory capacity and the biogeography of Crustacea at high southern latitudes. Polar Biol 33, 919–928 (2010). https://doi.org/10.1007/s00300-010-0768-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-010-0768-1